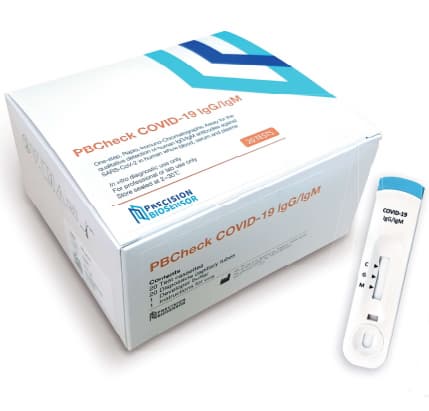
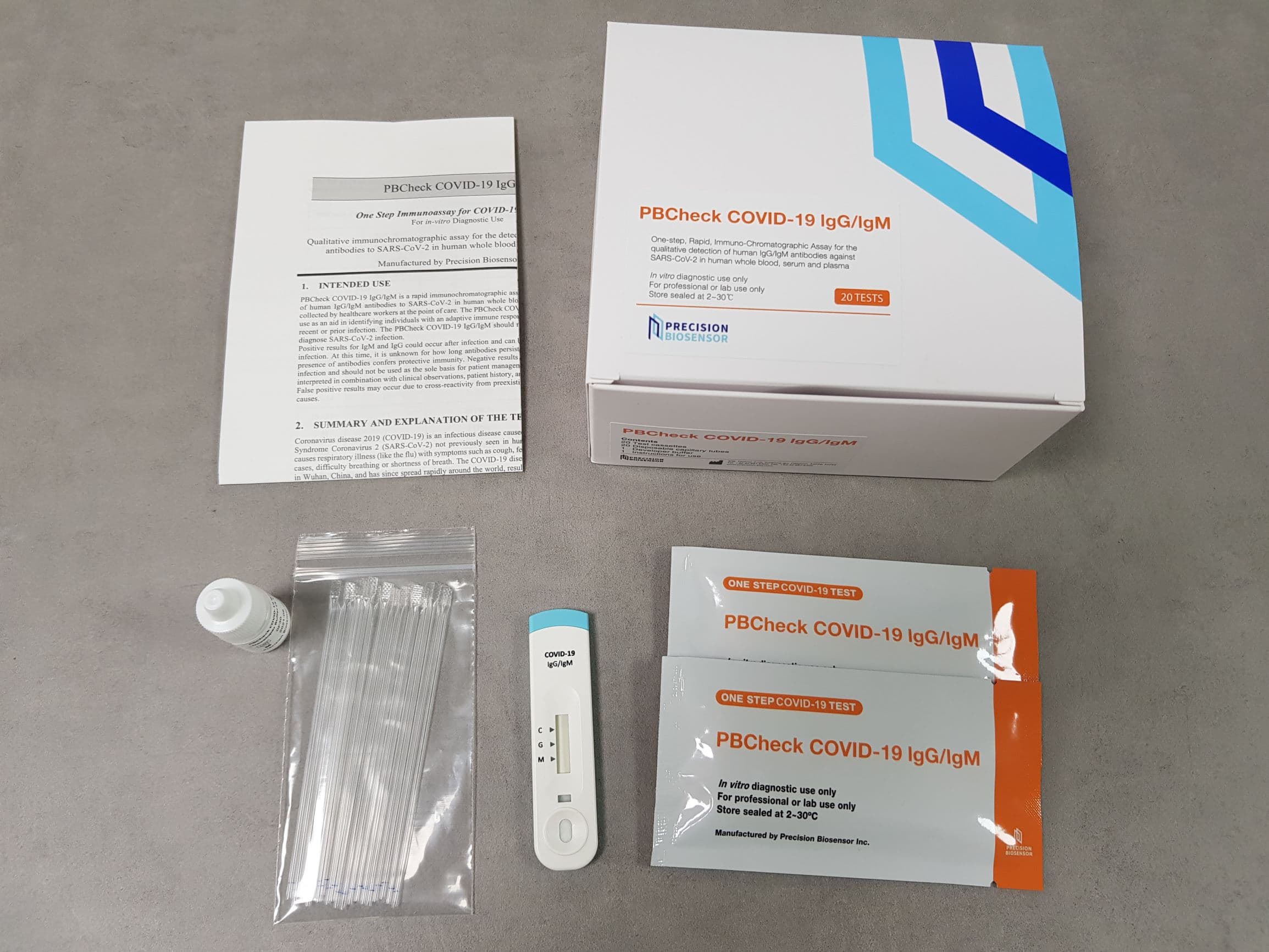
PBCheck COVID-19 IgG/IgM rapid chromatographic immunoassay
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
Precision Bio Co., Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
- Verified Certificate
-
5



| Product name | PBCheck COVID-19 IgG/IgM rapid chromatographic immunoassay | Certification | CE |
|---|---|---|---|
| Category |
Examination & Testing Instrument
Monitoring & Diagnostic Equipment |
Ingredients | - |
| Keyword | antibody , rapid , sars , covid | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
PBCheck COVID-19 IgG/IgM is a rapid immunoassay for the qualitative detection of human IgG/IgM antibodies to SARS-CoV-2
in human whole blood, plasma and serum sample. The PBCheck COVID-19 IgG/IgM Antibody Test is intended for use as an aid in identifying
individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.
Features
• Quick and Easy to use
• Fast results in 10 minutes
• No equipment required
• Multiple sample type
Application
Serology testing for COVID-19 can be used to identify whether people were previously infected by SARS-CoV-2 even if they haven’t shown
symptoms. This is important to determine, because the RT-PCR tests now being used to diagnose the coronavirus can detect the presence
of the viral material during infection, but it doesn’t indicate if the person was previously infected and subsequently recovered.
• Identify asymptomatic patients
• Help to verify quarantine release
• Rapid screening of suspected case
• Epidemiological tracing

Clinical performance
PBCheckCOVID-19 IgG/IgM Antibody Test | Comparator / RT-PCR | Total | ||
Positive | Negative | |||
Positive | IgM+/IgG+ | 35 | 0 | 35 |
IgM+/IgG- | 1 | 2 | 3 | |
IgM*/IgG+ | 16 | 0 | 16 | |
Negative | IgM-/IgG- | 3 | 98 | 101 |
Total | 55 | 100 | 155 | |
Positive Percent Agreement (PPA)= 52/55 (94.55%), 95% CI: 85.15% to 98.13%
Negative Percent Agreement (NPA)= 98/100 (98.00%), 95% CI: 93.00% to 99.45%
Test procedure

Interpretation of results

·IgM only positive: Appearance of pinkish red colored bands at the control line and
the IgM test line indicate that the SARS-CoV-2 IgM antibody was detected.
· IgG only positive: Appearance of pinkish red colored bands at the control line and
the IgG test line indicate that the SARS-CoV-2 IgG antibody was detected.
· IgM and IgG positive: Appearance of pinkish red colored bands at the control line,
the IgG test line and the IgM test line indicate that the SARS-CoV-2 IgG and IgM antibodies are detected.
· Invalid: If no colored line appears in the control region (C), the test results is invalid.
The test result is inconclusive, and assay should be repeated with a new test cassette.
Product Specification
Sample volume | 10μL Whole Blood 5μLSerum, Plasma |
Measuring time | 10 min |
Pack size | 20ea/box |
Shelf life | 12 months |
| Storage condition | Room Temperature (2-30℃) |
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
Precision Bio Co., Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
-
5



- President
- KIM HAN SIN
- Address
- 306 Techno 2 ro, Yuseong-gu, Daejeon, Korea
- Product Category
- Examination & Testing Instrument,Medical Test Kit,Monitoring & Diagnostic Equipment,Other Examination & Testing Instrumnet,Other Monitoring & Diagnostic Equipment
- Year Established
- 2015
- Company introduction
-
- Main Product