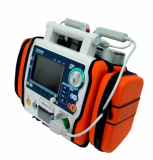
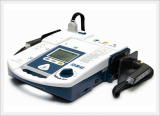
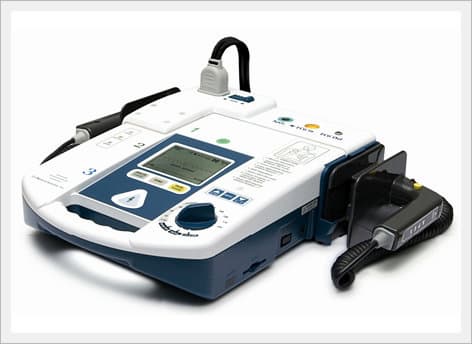
AED / Defibrillator (Paramedic CU-ER5)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Defibrillator

CU Medical Systems, Inc.
- Verified Certificate
-
17



| Product name | AED / Defibrillator (Paramedic CU-ER5) | Certification | - |
|---|---|---|---|
| Category | Defibrillator | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Paramedic CU-ER5 is an easy to use defibrillator/monitor with AED and manual modes plus SpO2 monitoring capaility and to be operated in manual mode with shocks delivered through external paddles or internal paddles.
Key Features
- Manual and AED Operation
- Defibrillation using external paddles or internal paddles
- Synchronized cardioversion
- Efficient and effective e-cube Biphasic technology (BTE Type)
- ECG monitoring (3 Lead ECG cable)
- SpO2 pulse oximetry with alarm
- CPR coaching
- Lightweight (about 2.8kg) and highly portable
- Versatile power supply
a. Rechargeable battery pack / Disposable battery pack
b. AC/DC adapter
c. Car cigar lighter jack - Intelligent data management system
a. Internal flash memory : 12 hours of event and ECG recording
b. SmartMedia card (32M) : 42 hours of event and ECG recording or 1 hour voice recording - External Link : UART port / IrDA port
- Automatic and operator initiated Self-Test
(Power on / Run time / Daily / Weekly / Monthly)
Specification
|
Model Name
|
Item Description
|
|
Paramedic CU-ER5
|
Defibrillator / Monitor
|
|
Parameter Description
|
Specified
|
| DEFIBRILLATOR | |
| Operating Modes | Semi automatic |
| Waveform | Biphasic (Truncated exponential type) |
| Shock Delivery |
Via multi-function defibrillator electrode pads |
| Reusable External Paddle | Yes |
| Pediatric Paddel | Yes |
| Multifunction Defi Pads(Disposable) | Yes |
| ECG Lead Select | I, II, III, aVR, aVL, aVF, V, Paddle/Pads, Ext ECG |
| Patient Impedance Range | 25 to 175Ohm |
| Charging Time | Less than 10 seconds |
| Energy sequence | Yes |
| Manual mode, J | 1~10, 15, 20, 30, 50, 70, 100, 120, 150, 170, 200 J |
| Synchronizer | Yes |
| Energy Selection | Mode/Energy Selector Knob |
| AED mode, J | Fixed energy at 150 Joules |
| Text and Voice Prompts | Yes |
| Protocol configured | Yes |
| ECG MONITOR | |
| ECG acquisition | Disposable electrodes |
| Monitors with ECG | Yes, 3 Lead ECG or 5 Lead ECG Cable |
| Input | Lead I, II, III (3 Lead ECG cable) |
| Lead I, II, III, aVR, aVL, aVF or V (5 Lead ECG cable) | |
| Electrodes | yes |
| ECG display | Yes |
| Type | High resolution display (Graphic LCD) |
| Message display | Yes |
| Heart rate display | Digital 30 to 300 bpm (±2 %) |
| ECG Size | 5, 10, 20mm/mV, Auto |
| Hear rate alarm | Less than minimum setting rate / Over than maximum setting rate |
| SpO2 Pulse Oximetry | |
| SpO2 monitoring | Yes (Nellcor) |
| Saturation | 70~100% (±3digits) |
| Pulse Rate | 20~250bpm (±3bpm) |
| Perfusion | 0.20% |
| DATA STORAGE | |
| Internal Memory | Yes |
| Information stored | Analysis/events |
| Internal memory | Yes |
| Event & ECG record | Yes |
| Database storage | Yes |
| Capacity, hr | 12 hours |
| External Memory | Yes |
| Information stored | Analysis/events |
| Internal memory card | Yes |
| Event & ECG record | Yes |
| Voice recording | Yes |
| Database storage | Yes |
| Capacity, hr | 42 hours (Event & ECG) or 1 hour (Voice Recording) |
| EXTERNAL LINK | |
| Data management | Yes |
| Software | Yes(CU-Expert) |
| UART | Yes |
| IrDA | Yes |
| External Thermal Printer | Yes |
| Real-time Printer | Yes |
| BATTERY | |
| Type | 4500mAh, 12V, rechargeable, Nickel Metal Hydride |
| Integral /removable | lntegral |
| Charging method | AC adapter / Car Cigar Lighter Jack / both |
| Charge time, hr | 4 ot 5 hours |
| Battery Indicators | Battery level indicator / Battery charging indicator |
| AC ADAPTER | |
| Input | 100 ~ 240V AC 50 / 60Hz 1.2A |
| Output | 12V DC 3.5A |
| PHYSICAL | |
| Size | 254 X 365 X 105mm (Without external paddle) |
| 455 X 365 X 105mm (With external paddle) | |
| Weight | 4.7Kg (with external paddle) |
| DISPLAY | |
| Type | High resolution display (Graphic LCD) |
| Size | 4 inches (10.16cm) diagonal |
| Resolution | 320 X 240 pixels |
| Sweep Speed | 25mm/s nominal, stationary trace, sweeping erase bar |
| Viewing Time | 3.2 seconds |
| AUTOMATIC SELF-TEST | |
| Power on Self-Test / Run Time Self-Test | Yes |
| Manual Self-Test | Yes |
| Periodic Self-Test | Daily / Weekly / Monthly |
| PACKAGE CONTENTS | |
| Device | Yes |
| Reusable External Paddle - Pediatric Included | Yes |
| 3 Lead ECG Cable | Yes |
| Power Cord | Yes |
| AC Adapter | Yes |
| Internal Rechargeable Battery Pack | Yes |
| Operator's Manual | Yes |
| OPTIONAL ACCESSORIES | |
| Multifunction Defi Pads (Disposable) | YES |
| Adapter for Defi Pads | YES |
| 5 Lead ECG Cable | YES |
| ECG Electrodes (50EA) | YES |
| External Thermal Printer and Paper | YES |
| Cigar Lighter Jack for Car | YES |
| External memory card | YES |
| Software with UART cable | YES |
| SpO2 Module (MP100, Finger Probe, Extension Cable) | YES |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Harock Na
- Address
- 5F, Cheonggye Plaza, 991-4 Cheonggye-dong, Euiwang-si, Gyeonggi-do, Korea
- Product Category
- Defibrillator
- No. of Total Employees
- 101-500
- Company introduction
-
CU Medical Systems, Inc. was established in 2001, and we have been in this business for more than 10 years.
CU Medical Systems, Inc. is a Leader of Medical Devices specialized in defibrillators involved in the research, development, manufacturing and customer service for all its products with the latest IT technology targeting domestic and overseas market.
Our products are enjoying strong sales in Korea and have been exporting to about 70 countries around the world since 2001. We are the only company in Korea covering a full range of defibrillators from AED for the public places to Defibrillator/Monitor for the hospitals.
The products of the CU Medical Systems, Inc. are designed and manufactured according to international standards. All of our products got all certifications such as ISO, CE, Korean FDA, USA FDA and SFDA.
If you visit our website, www.cu911.com, you will find out all of our product information in detail.
We trust that you will pay prompt attention to this matter and that a mutually profitable long lasting business relation between us will be realized soon.
- Main Markets
-
 France
France
 Germany
Germany
 Italy
Italy
 Lebanon
Lebanon
 Spain
Spain
- Main Product
Related Products
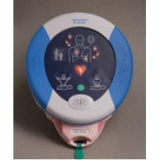
HeartSine Pediatric-Pak PAD (Public Access Defibrillator)
Defibrillator

Defibrillator
Automated External Defibrillator, AED, HeartResQ

HeartPlus NT-180


































 South Korea
South Korea