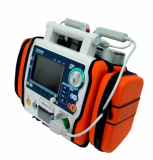
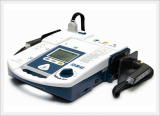
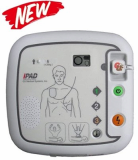
Defibrillator, AED (CU-HD1)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Defibrillator

CU Medical Systems, Inc.
- Verified Certificate
-
17



| Product name | Defibrillator, AED (CU-HD1) | Certification | - |
|---|---|---|---|
| Category | Defibrillator | Ingredients | - |
| Keyword | defibrillator , biphasic defibrillator , defibrillator machine , defibrillator monitor | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
1. Product overview
LifeGain CU-HD1 – this is the company’s high end defibrillator/monitor intended for hospital use. It has defibrillation capabilities in AED and manual modes and may use disposable electrode pads and external and internal paddles for shock delivery. It has a transcutaneous pacer for temporary pacing of patients with low heart rate. It has an SPO2 module for the monitoring of oxygen saturation in the patient’s blood and a 12-lead ECG monitor for the evaluation and monitoring of the patient’s ECG.
It has a memory card port for the storage of acquired signals and an integrated printer for the generation of hard copies of the patient’s physiological signals. These features make this defibrillator suitable for use inside a hospital. This product is a defibrillator designed for qualified emergency staff and medical professionals to deliver defibrillating shocks to victims of sudden cardiac arrest and restore the normal ECG rhythm.
* AED Mode
AED Mode is delivered to patients who are exhibiting the symptoms of Sudden Cardiac Arrest (SCA) including ventricular fibrillation, ventricular tachycardia, in order to bring them back to life by delivering electric shock and restoring the normal ECG rhythm.
In the AED mode, defibrillation pads must be used. These defibrillation pads work to obtain the ECG signal of patients and deliver electric shock according to the patients' condition.
* Manual Mode
Manual Mode is divided into two functions such as asynchronous defibrillation and synchronous cardioversion. In asynchronous defibrillation, the usage target is the same in AED Mode.
For the asynchronous defibrillation treatment in the manual mode, the user is able to choose the level of electric shock energy from the range of 1-200 joule by the use of pads or paddles.
On the other side, when used for synchronous cardioversion, it is used on patients with the symptoms of atrial fibrillation. It is designed to analyze the R-wave of ECG QRS and deliver R-wave synchronized electric shocks.
In the Manual mode, synchronous cardioversion treatment can be delivered to patients with rapid atrial fibrillation, ventricular tachycardia, and cardiac ischemia.
* Pacer Mode
Pacing is a method applied to patients who had lost natural cardiac movement functions, mostly used on patients with bradycardia. LiFEGAIN CU-HD1 functions to support non-invasive pacing, a way of helping maintain a patient’s pulse by attaching its electrode to the patient’s skin and delivering artificial electric stimulation to the heart. Pacing mode is divided into the 'Fixed mode' and the 'Demand mode'.
* Monitoring Mode
Patient monitoring mode features the ECG monitoring function and the function to measure the level of SpO2, functional oxygen saturation in the blood.
For the ECG monitoring function, you can discriminatingly use the 3-lead, 5-lead, 12-lead ECG cables. During the patient monitoring session, it is possible to analyze the ECG results to use the alarming function according to the conditions such as the number of pulses, ventricular fibrillation or ventricular tachycardia etc. If using an ECG cable, convert "Rotary Switch" to "Monitor mode" before usage.
* Key features
- Manual and AED Operation
- Defibrillation using paddles or pads
- Synchronized cardioversion
- Efficient and effective e-cube Biphasic technology (BTE Type)
- SpO2 pulse oximetry with alarm
- ECG monitoring (3 Lead ECG / 5 Lead ECG / 10 Lead ECG)
- Noninvasive pacing mode
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Harock Na
- Address
- 5F, Cheonggye Plaza, 991-4 Cheonggye-dong, Euiwang-si, Gyeonggi-do, Korea
- Product Category
- Defibrillator
- No. of Total Employees
- 101-500
- Company introduction
-
CU Medical Systems, Inc. was established in 2001, and we have been in this business for more than 10 years.
CU Medical Systems, Inc. is a Leader of Medical Devices specialized in defibrillators involved in the research, development, manufacturing and customer service for all its products with the latest IT technology targeting domestic and overseas market.
Our products are enjoying strong sales in Korea and have been exporting to about 70 countries around the world since 2001. We are the only company in Korea covering a full range of defibrillators from AED for the public places to Defibrillator/Monitor for the hospitals.
The products of the CU Medical Systems, Inc. are designed and manufactured according to international standards. All of our products got all certifications such as ISO, CE, Korean FDA, USA FDA and SFDA.
If you visit our website, www.cu911.com, you will find out all of our product information in detail.
We trust that you will pay prompt attention to this matter and that a mutually profitable long lasting business relation between us will be realized soon.
- Main Markets
-
 France
France
 Germany
Germany
 Italy
Italy
 Lebanon
Lebanon
 Spain
Spain
- Main Product
Related Products
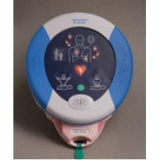
HeartSine Pediatric-Pak PAD (Public Access Defibrillator)

Automatic Endoscope Washer-disinfectors(CYW-201)

Automatic Endoscope Washer-disinfectors (CYW-100)
Zoll Propaq MD

HeartPlus NT-180


































 South Korea
South Korea