MONALISA High Elastic
Volume Effect and Retention - The uniform sized hyaluronic acid particles with optimal viscoelasticity can maintain a long-lasting volume.
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others
- Production method
- OBM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- antiaging, filler, dermafiller
- Category
- Cosmetic Raw Materials
GENOSS CO., LTD.
- Recent Visit
- Dec 23, 2024
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
17


| Product name | MONALISA High Elastic | Certification | - |
|---|---|---|---|
| Category | Cosmetic Raw Materials | Material | - |
| Keyword | antiaging , filler , dermafiller | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 1000 |
| Supply type | OBM | HS code | - |
Product Information
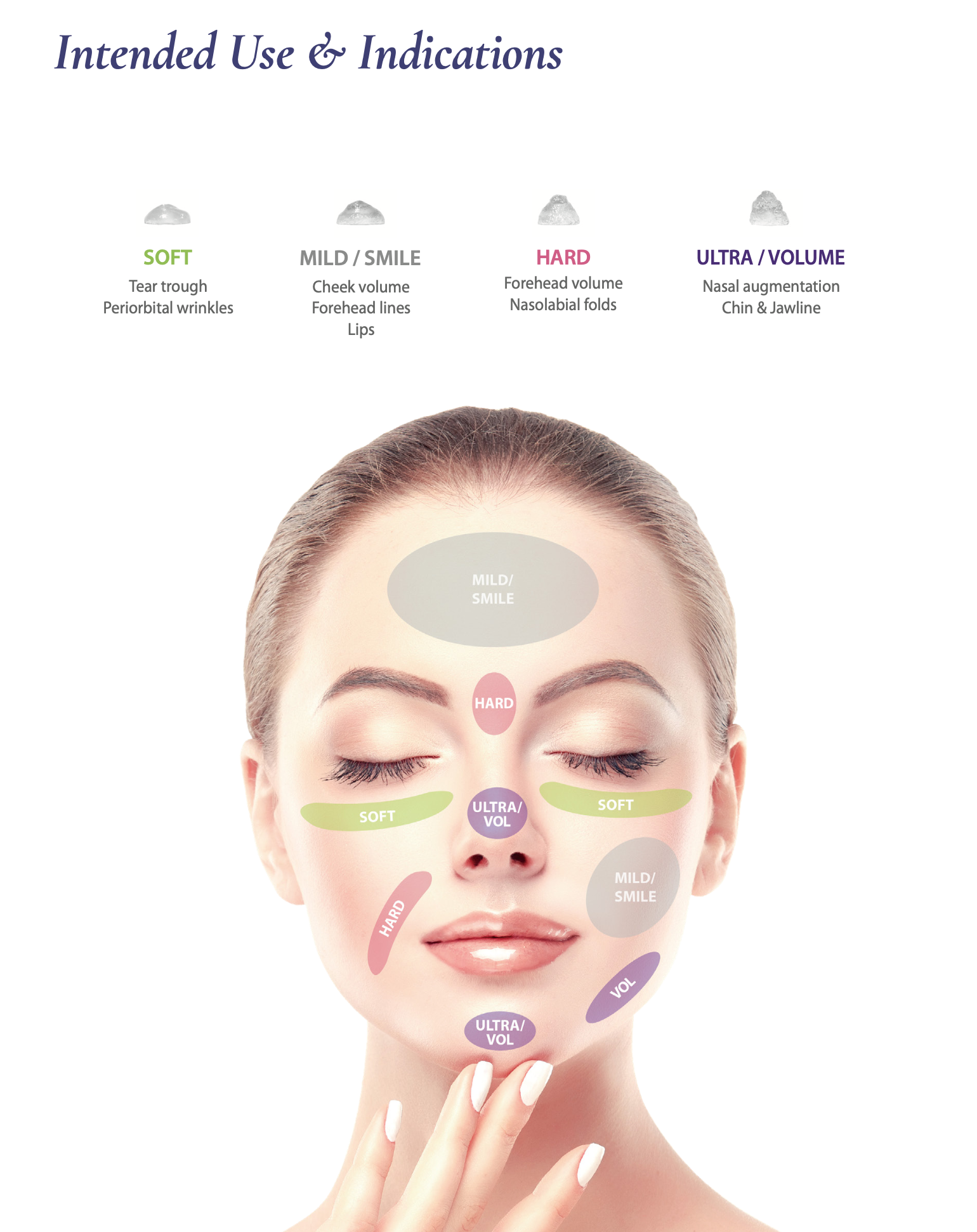
- Wrinkles with crosslinked hyaluronic acid particles temporarily
- Made with pharmaceutical grade hyaluronic acid
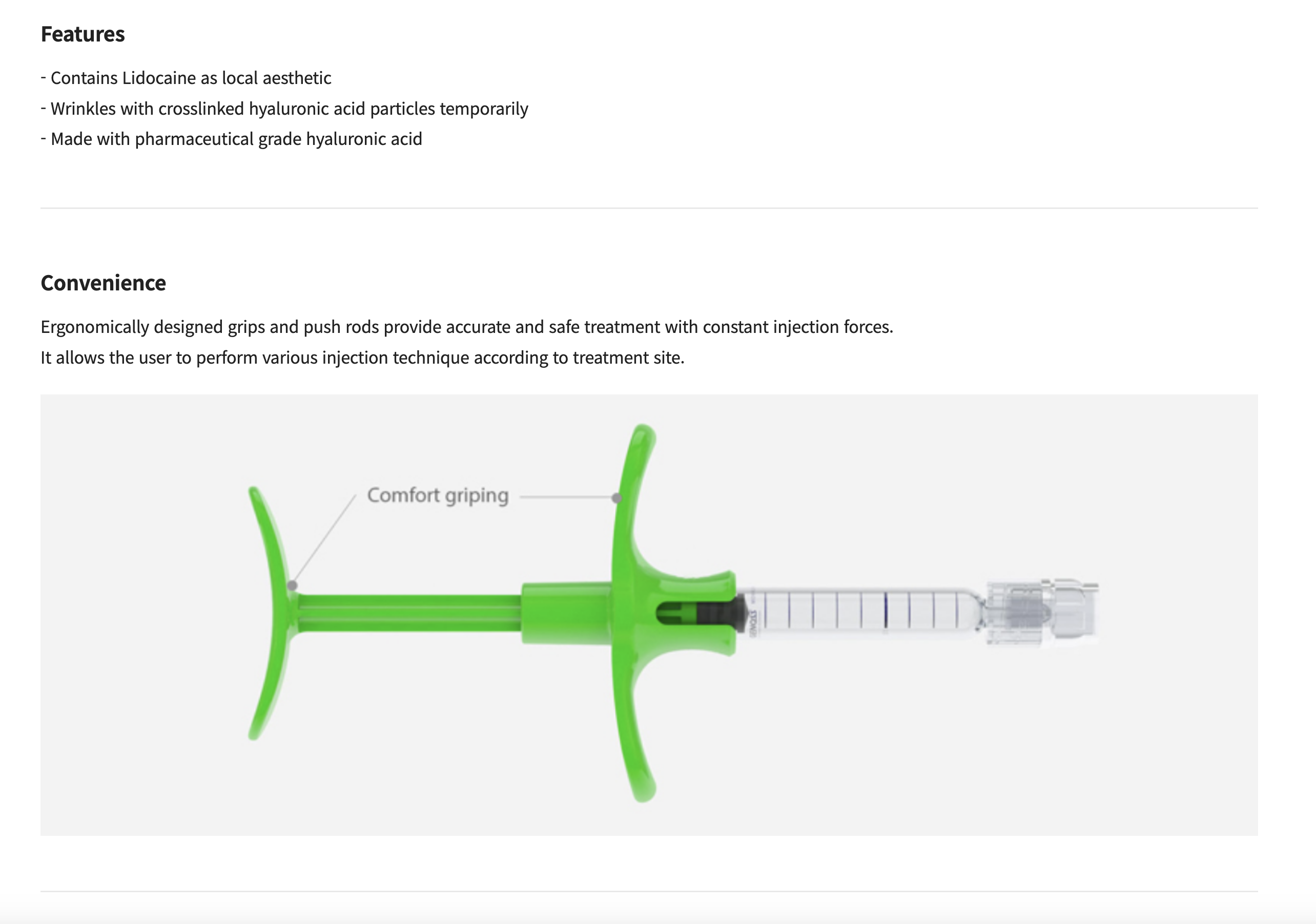
It allows the user to perform various injection technique according to treatment site.
The residual amount of BDDE is strictly regulated to less than 2ppm. It was not detected in Every MONALISA Filler.

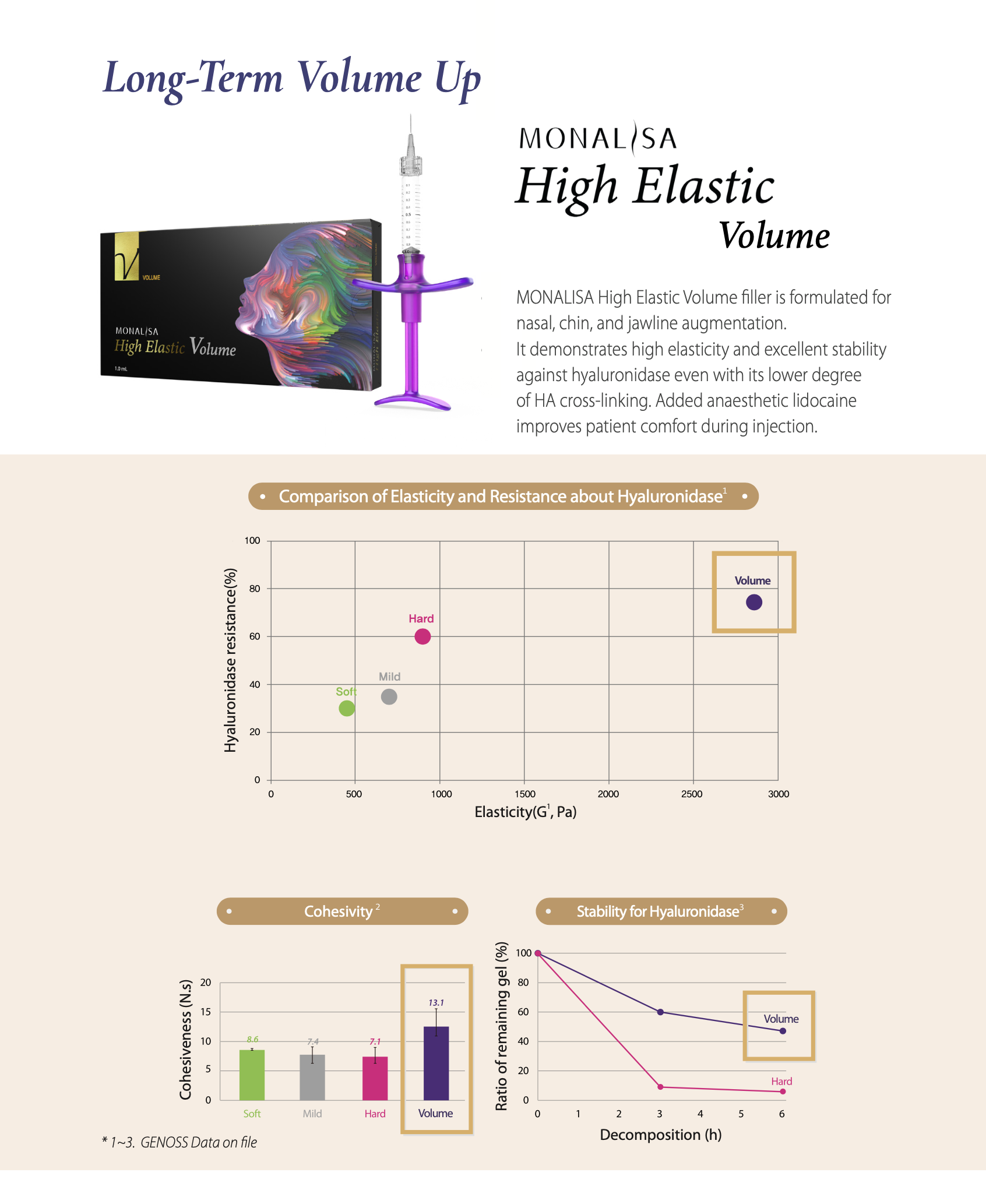
1. Volume Effect and Retention
The uniform sized hyaluronic acid particles with optimal
viscoelasticity can maintain a long-lasting volume.
2. Safe to Use on Patients
MONALISA is safe to use on patients due to low level
of endotoxin and essentially no BDDE residue.
3. Easy Procedural Operation
The ergonomically-designed rod and grip allow the even distribution of pressure during injection to enable an accurate and safe treatment for both the clinician and the patient.
4. Highly Pure Hyaluronic Acid
GENOSS implements a strict quality control system through direct involvement
in the entire production process from the base material of hyaluronic acid to the final product.
5. Global Standard Quality Control
To guarantee the quality of MONALISA, GENOSS strictly fulfills and complies
with the international quality regulations, including KGMP, ISO 13485 and ISO 9001.
B2B Trade
| Price (FOB) | Negotiable | transportation | Express |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
GENOSS CO., LTD.
- Country / Year Established
-
 South Korea
/
South Korea
/
- Recent Visit
- Dec 23, 2024
- Business type
- Others
-
17


- President
- Chung sung min
- Address
- R&DB Center Iui-dong, Suwon Si Yeongtong-gu, GYEONGGI-DO, 443-766KOREA
- Product Category
- Other
- No. of Total Employees
- 1-50
- Main Product
Related Products
Paeonia root extract (natural anti-microbial, anti-oxidant material)

100% Natural Spongilla lacustris

Neohesperidin

Limonin

Pvp K30