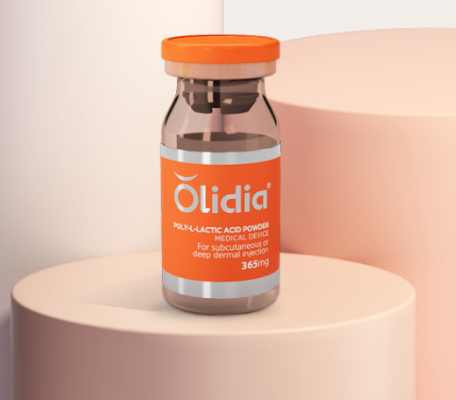
Olidia PLLA Filler (Poly L-Lactic Acid Powder) dermal filler
dermal filler, plla, plla filler, poly l lactic acid
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others,Western Union
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- dermal filler, plla, plla filler, poly- l- lactic acid powder
- Category
- Other Beauty Products

Vizion Co., Ltd.
- Verified Certificate
-
2

| Product name | Olidia PLLA Filler (Poly L-Lactic Acid Powder) dermal filler | Certification | - |
|---|---|---|---|
| Category | Other Beauty Products | Material | - |
| Keyword | dermal filler , plla , plla filler , poly- l- lactic acid powder | Unit Size | 3.0 * 3.0 * 7.0 mm |
| Brand name | - | Unit Weigh | 365 mg |
| origin | South Korea | Stock | 1000 |
| Supply type | Available | HS code | - |
Product Information
Olidia ®
Injectable Poly L-Lactic Acid Powder
Helps you recharge loose collagen and achieve natural volume.
Olidia is a polyelactic acid suspension.Inject into deep dermis or subcutaneously
The facial area of an adult through physical restoration
Nasolabial folds Temporarily improves.
Package:365mg*10vials/piece
Device Classification : Graft/prosthesis
Storage : Store at room temperature below 30℃. It is forbidden to be frozen.
Country of manufacture: South Korea
Translation resulMade in koreWith GMP ISO CE certificate
Olidia® is biodegradable dermal filler. Poly L-Lactic Acid powder which is an ingredient approved by U.S. FDA. It is safe for human body because PLLA is biodegradable and biocompatible. Injected Olidia starts stimulating collagen generation inside the skin.Stimulated collagen boost face volume and the skin gets filled with natural volume.
*Main point
Olydia Booster is a collagen booster made with PLLA, which has the same ingredients as Sculptra, but has a softer form, reducing the likelihood of lumps or nodules forming in the treatment area. The PLLA ingredient is FDA approved and has been used for a long time for its volumizing effect.
1.Olydia is a medical device approved by the Korea Ministry of Food and Drug Safety.
2.Olydia has received CE certification, certifying that it has been produced based on manufacturing that meets the standards for safety, health, and environment set by European countries.
3.Olydia is a medical device manufactured and managed in a GMP-certified facility.
4.Olydia temporarily improves wrinkles in the facial nasolabial area.
*How to use :
Before Olidia treatment, you should following correct guide line.
You need to dilute the solution above all.
The threatment with this product should be performed by the linear threading technique.
*After care
1.For 5 days after the injection, pls massage gently for 5 minutes, 5 times a day.
2.After the injection, You can wrap the ice pack in a towel to cool the treatment area and reduce swelling.
3.Excessive exposure to sunlight and ultraviolet rays should be avoided, and sunscreen is recommended.
4.After the injetion, you must avoid saunas and stop drinking and smoking for about a week.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,Western Union | Shipping time | Negotiable |

- President
- Seonghee Kim
- Address
- , Gangnam-gu, Seoul, Korea
- Product Category
- Other Beauty Appliance
- Year Established
- 2020
- Company introduction
-
- Main Markets
-
 Iran
Iran
 Malaysia
Malaysia
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product
Related Products
Botalinum Ampoule

Ovaco B.P Cell Ampoule
Yuvenis Skin Booster Salmon DNA PN
![[크기변환]데일리차이_비타민멀티필터샤워기이미지](https://web.tradekorea.com/product/18/2113018_02/Multi_filtered_ACF___Vitamin_Shower_Capsule_filter_Shower_head_2.jpg)
Vitamin Multi filtered Shower head(US CEC Certified)

skin79 Water Wrapping Moist Cool Sun Stick SPF50+ PA++++


































_dermal_filler_2.png)
 South Korea
South Korea
_dermal_filler_2.png)


