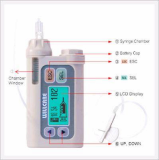
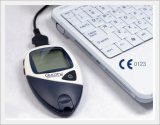
GluNeo Lite Smart
This blood glucose measuring biosensor has acquired US FDA and CE approval and needs only a 0.5㎕ blood sample to report accurate blood glucose levels.
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Glucose Meter

OSANG HEALTHCARE CO., Ltd
- Verified Certificate
-
16





| Product name | GluNeo Lite Smart | Certification | FDA , CE |
|---|---|---|---|
| Category | Glucose Meter | Ingredients | - |
| Keyword | bluetooth , diabetes , bgms , ivdr | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 5000 |
| Supply type | ODM,OEM | HS code | 9018199000 |
Product Information
The GluNEO® Lite Smart Blood Glucose Monitoring System is intended for use outside the body (invitro diagnostic use only). It should be used only for testing blood glucose with fresh whole blood samples(Capillary and/or venous). It should not be used for the diagnosis of diabetes. Consult with your physician or diabetic healthcare professional about daily management of your diabetes and proper use of the glucometer. Please pay close attention when handling blood. Improper procedures may cause serious hazards to your health. The GluNEO® Lite Blood Glucose Monitoring System contains small parts, which could be a choking hazard for children if swallowed.
| Sample Type | Capillary and/or venous whole blood |
| Sample Volume | 0.5 ul |
| Test Range | 20~600mg/dL |
| Reading Time | 5 Seconds |
| Calibration | Plasma-equivalent |
| Hematocrit | 20~65 % |
| Altitude | 3048 meter Up to(10,000 feet) |
| Operating Temperature | 10~40 ℃ |
| Operating Humidity | 10~90% |
| Test Strip Storage Temperature | 2~30 ℃ and no direct sunlight. Do not Freeze |
| Display Type | LCD |
| Power Source | 3V Li Battery(CR2032) X 1 |
| Battery Life | One year after purchasing |
- Verified Certificate
-


B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

OSANG HEALTHCARE CO., Ltd
-
16





- President
- SeunEuk Hong
- Address
- 132 Anyangcheondong-ro, Dongan-Gu, Dongan-gu, Anyang-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit
- Year Established
- 1996
- No. of Total Employees
- 101-500
- Company introduction
-
OSANG Healthcare has been a leader in the Korean IVD (In-Vitro Diagnosis) industry since its founding in 1996, ever expanding a wide array of medical devices for biochemical, immunoassay, molecular diagnostics, and digital healthcare.
OSANG Healthcare Co., was the first South Korean SARS CoV 2 test kit producer to receive authorization from the U.S. Food & Drug Administration in April 2020.
The company has supplied countries all over the world and has already sold 760,000 tests to US customers, including the Federal Emergency Management Agency, Agency (FEMA). Giving the first priority to our customers and partners, we will further develop powerful synergies with other affiliates in the group.
- Main Markets
-
 China
China
 Germany
Germany
 Greece
Greece
 India
India
 Indonesia
Indonesia
 Iran
Iran
 Italy
Italy
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
 Yemen
Yemen
- Factory Information
-
Osang Healthcare
- Main Product
- Attached File



































 South Korea
South Korea