BluLink
Track and organize your glucose test results uploaded from your Osang Healthcare's Glucose Monitoring System.
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Analyzer

OSANG HEALTHCARE CO., Ltd
- Verified Certificate
-
17





| Product name | BluLink | Certification | - |
|---|---|---|---|
| Category | Medical Analyzer | Ingredients | - |
| Keyword | cholesterol analyzer , smartphone , blood pressure meter , bluetooth blood glucose meter | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 1 |
| Supply type | Available | HS code | - |
Product Information
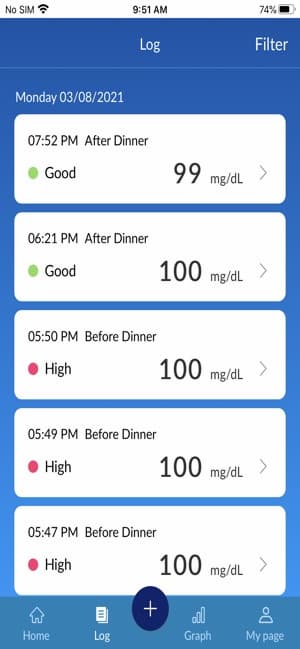
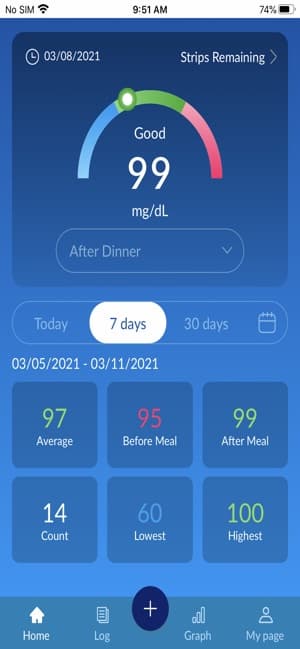
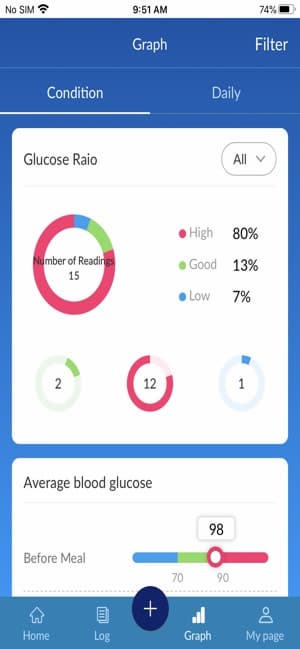
The BluLink application will track and organize your test results uploaded from your Osang Healthcare Glucose Monitoring System and allow you to see the time of day you tested. With the click of single button on you meter, you can upload your recent test results to the BluLink application. The BluLink application will then organize based upon your testing goals!
BluLink Application Features:
- 7-Day, 30-Day, 3 Month or Custom of blood sugar Log
- Automatically organizes your results when uploading from you EasyTouch . - BluLink Blood Glucose Monitoring System
- Built-in charts and graphs to view your testing over time.
- Color-coded readings to distinguish high, low, and in range readings.
- Customizable testing goals to accommodate your testing needs.
- Test strip tracker to help you know how many test strips you have left.
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

OSANG HEALTHCARE CO., Ltd
-
17





- President
- SeunEuk Hong
- Address
- 132 Anyangcheondong-ro, Dongan-Gu, Dongan-gu, Anyang-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit
- Year Established
- 1996
- No. of Total Employees
- 101-500
- Company introduction
-
OSANG Healthcare has been a leader in the Korean IVD (In-Vitro Diagnosis) industry since its founding in 1996, ever expanding a wide array of medical devices for biochemical, immunoassay, molecular diagnostics, and digital healthcare.
OSANG Healthcare Co., was the first South Korean SARS CoV 2 test kit producer to receive authorization from the U.S. Food & Drug Administration in April 2020.
The company has supplied countries all over the world and has already sold 760,000 tests to US customers, including the Federal Emergency Management Agency, Agency (FEMA). Giving the first priority to our customers and partners, we will further develop powerful synergies with other affiliates in the group.
- Main Markets
-
 China
China
 Germany
Germany
 Greece
Greece
 India
India
 Indonesia
Indonesia
 Iran
Iran
 Italy
Italy
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
 Yemen
Yemen
- Factory Information
-
Osang Healthcare
- Main Product
- Attached File



































 South Korea
South Korea