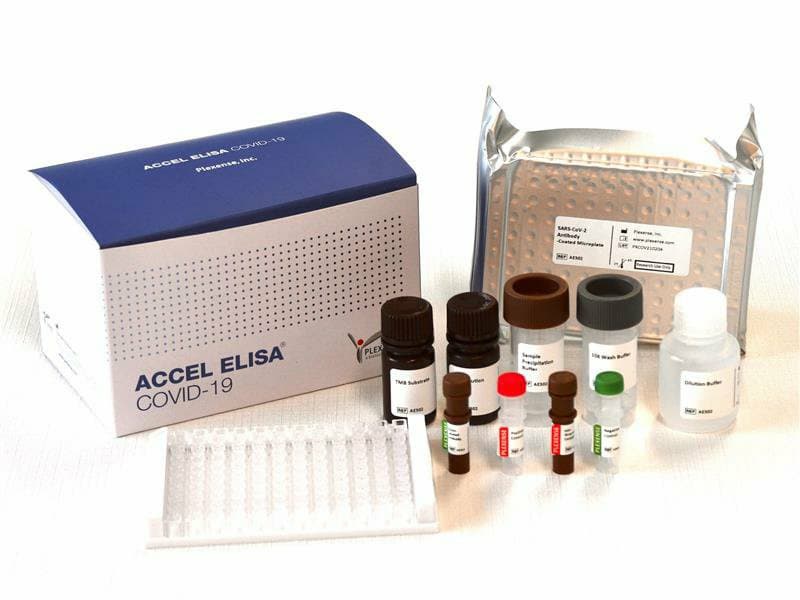
ACCEL ELISA COVID-19 Saliva Antigen Kit
Rapid ELISA for Detection of SARS-CoV-2 Antigen in Human Saliva
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- AE302
- Payment Terms
- L/C,T/T
- Production method
- Available,ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable

Plexense
- Verified Certificate
-
5




| Product name | ACCEL ELISA COVID-19 Saliva Antigen Kit | Certification | CE |
|---|---|---|---|
| Category | Other Examination & Testing Instrumnet | Material | - |
| Keyword | antigen , detection , saliva , covid-19 | Unit Size | 158.0 * 93.0 * 103.0 mm |
| Brand name | AE302 | Unit Weigh | 250 mg |
| origin | South Korea | Stock | 0 |
| Supply type | Available,ODM,OEM | HS code | 382200 |
Product Information
ACCEL ELISA® COVID19 Saliva Antigen test is a rapid ELISA for the qualitative detection of neucleocapsid protein (antigens) of SARS-CoV-2 present in saliva sample.
This product is especially for the countries that lack infrastructure for a nasopharyngeal PCR test method which is called the "Gold Standard" worldwide, or a place that is having inconvenient by visiting lab or facility for the test.
Fast and Reliable Sample Collection
ACCEL ELISA® COVID-19 Saliva Antigen test can be deployed at on site collection centers for hospital staff, patients and visitors, remote collection facilities for mass testing of local populations or in dedicated, pop-up labs at airports or universities.
Rapid and Scalable for Mass Testing
The ACCEL ELISA® testing system offers simple and easy scaling up of testing capacity to meet surging requirements.
Low Cost
ACCEL ELISA® COVID-19 Saliva Antigen test also offers a number of cost saving advantages over other test kits that require the usage of certified swab and collection receptacle. It also does not have to be obtained by a medically trained personnel, significantly reducing mass screening costs.
Performance (Accuracy)
The ACCEL ELISA® COVID19 Saliva Antigen test kit showed
91.7% of Sensitivity and 97.1% of specificity, relative to the result of RT-PCR.
Specification
Specimen: 30~50μL of saliva
Testing time: 95 mins (1.5 hrs)
Packaging unit: 96 Tests/Kit (92 samples)
Storage condiotion: 2 – 8°C
※ Product package contains 1 well-plate and 9 reagents
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,T/T | Shipping time | Negotiable |

- President
- KIM GI BEOM
- Address
- 17015, Giheung-gu, Yongin-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit
- Year Established
- 2012
- No. of Total Employees
- 1-50
- Company introduction
-
We are Plexense Inc., a South Korean company that specializes in the development and production of a fast and cost-effective ELISA immunoassay platform for clinical, food and life science testing.
Currently we are focusing on diagnostic kits for the COVID-19.
Our kit is an advanced ELISA platform which is very cost-effective, patented technology that makes the accuracy of our product excellent.
If you have more questions about our diagnostic kit for the Covid-19, Please don’t hesitate to contact us or you also can visit our website: www.plexense.com to register your interest or review our product information.
- Main Markets
-
 Brazil
Brazil
 Canada
Canada
 Colombia
Colombia
 India
India
 Indonesia
Indonesia
 Pakistan
Pakistan
 Philippines
Philippines
 South Africa
South Africa
- Main Product



































 South Korea
South Korea

Medium_for_collecting_corona_2.jpg)




