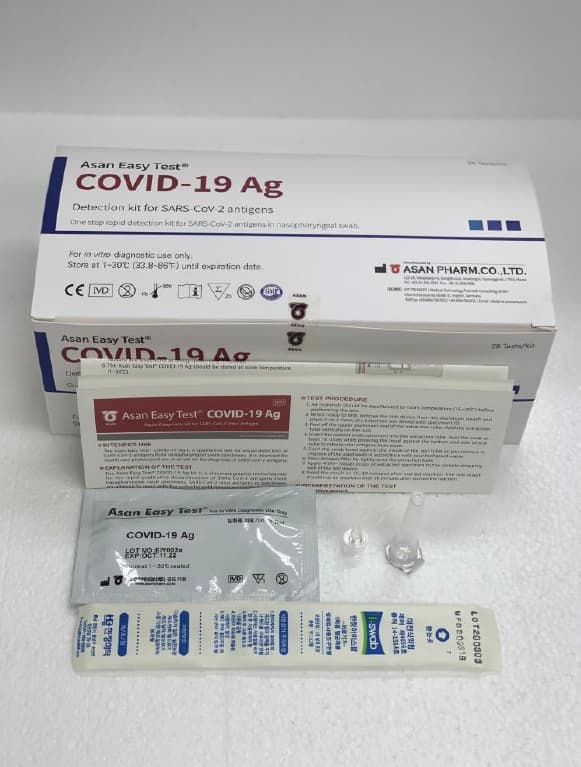
Asan Easy Test COVID-19 Ag
Asan Easy Test COVID-19 Ag
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Asan Easy Test COVID-19 Ag
- Payment Terms
- L/C
- Production method
- ODM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, rapid test kits, test kits
- Category
- Medical Test Kit
Aju Corporation
- Country / Year Established
-
 South Korea
/
1960
South Korea
/
1960
- Business type
- Others
- Verified Certificate
-
6


| Product name | Asan Easy Test COVID-19 Ag | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Material | - |
| Keyword | corona virus , rapid test kits , test kits | Unit Size | 25.0 * 12.5 * 9.0 cm |
| Brand name | Asan Easy Test COVID-19 Ag | Unit Weigh | 456 g |
| origin | South Korea | Stock | 4000 |
| Supply type | ODM | HS code | - |
Product Information
1. Product name
Product Name Rapid detection kit for SARS-CoV-2 antigens
Model Name Asan Easy Test® COVID-19 Ag
Catalogue Number AM3474-K (20T) / AM3476-K (25T)
2. Package unit
Self-package unit: Refer to external (package box) labeling
Package unit
Test device 20EA/Kit 25EA/Kit
Extraction buffer 20EA(0.35mL)/Kit 25EA(0.35mL)/Kit
Dropper filter tip 20EA/Kit 25EA/Kit
Sample collection swab 20EA/Kit 25EA/Kit
3. Purpose of use
Asan Easy Test® COVID-19 Ag is an in vitro diagnostic medical device
that diagnoses COVID-19 by qualitatively testing SARS-CoV-2 antigen in
nasopharyngeal swab by immunochromatographic assay (ICA).
1) Specimen preparation and storage
(1) Specimens: nasopharyngeal swabs
① To obtain a nasopharyngeal swab, use the sample collection swab
included in the kit.
② For nasopharyngeal swabs collection, it is recommended to insert the
swab into the nasal cavity where the most secretions are generated,
and rotate it once carefully to collect the secretion enough to be
visibly wet.
(2) Specimen storage
It is recommended to use the specimen immediately after collection, if
possible. If it cannot be tested immediately after collection, put it in a
lidded storage container. Specimens contained in Viral Transport Media
can be stored for up to 72 hours when stored refrigerated (2~8℃) or
frozen (-20℃).
2) Preparation process before test
Before testing, place the test device, extraction buffer, and specimen at
room temperature, and shake extraction buffer gently before use. The most
suitable temperature condition for the test is room temperature (15~25℃).
If the reagent is stored at room temperature, it can be opened and used
immediately.
3) Test procedure
(1) Place the patient’s swab sample in a tube containing the extraction
buffer and rub it against the wall of the tube and rotate at least 8 to 10
times to allow for sufficient extraction.
(2) The extracted swab is pulled out by turning it along the wall of the tube.
During this process, the extract from the swab is squeezed out.
(3) Used swab is classified as infectious waste and disposed of safely.
(4) After combining the extraction buffer tube with the dropper filter
tip, take out the device from the aluminum pouch and add 4 drops
(90~100μl) to the sample well (S).
(5) If the control line (C) is clearly visible after 15-20 minutes, the result is
read.
(6) As the reaction time increases, the color band of the control line (C) and
the test line (T) may become darker. Therefore, it is possible to obtain
more accurate results by determination at a constant time after starting
the reaction. When reacting for a long time, non-specific reactions may
occur, so results after 30 minutes are not used for determination.
4) Quality control
All test results should have a colored band on the control line (C).
*more details in attached file.
1.Kit size
25*12.5*9 cm
2.The number of tests in kits
25 Tests
3.Kit Gross weight
0.35 kg
4.Outer Carton size
63*49*42 cm
5.Outer Carton Gross weight
11.40 kg
6.Kits per each carton (Max)
25 Kits (625Tests)
Performance evaluation
1) Analytical sensitivity (Limit of detection, LoD)
When tests were repeated twenty times with diluted inactivated SARSCoV-
2, the lowest concentration at which more than 95% of the total tested
number could be judged as positive was determined as the LoD. In result,
the LoD of isolate USA-WA1/2020, isolate Italy-INMI1 and isolate Hong
Kong/VM20001061/2020 are 5.90×102 TCID50/mL, 7.46×103 TCID50/mL and
1.23×103 TCID50/mL, respectively.
*more details in attached file
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C | Shipping time | Negotiable |
- President
- Park Sang Il
- Address
- 351 Gangnamdaero Seochogu, Gangnam-gu, Seoul, Korea
- Product Category
- Construction Materials Processing
- Year Established
- 1960
- No. of Total Employees
- 101-500
- Company introduction
-
Aju Corporation, established in 1960, manufactures various construction materials. It has been
stretching itself as a industry leader and has continued to grow on a scale of 957 million USD
of total assets, 314 million USD of sales and 25 million USD of operating profit at the end of
December 2011.
We established overseas subsidiaries in Vietnam(Ho Chi Minh City) and Cambodia(Phnom Penh)
which is a PHC pile factory and concrete telegraph pole factory in each.In addition, we are carrying out international trade to expand our business such as tire, steel and lumber industry. Also we handle various products for new export business and we are in the process of producing competitive products in various fields such as home&interior(kitchen utensils).
- Main Markets
-
 Cambodia
Cambodia
 South Korea
South Korea
 Viet Nam
Viet Nam
- Main Product
Related Products

Covid 19 Detection
AFP/PSA/CEA Rapid Test
BioTracer Cardiac 3 in 1 Rapid Test
BioTracer Troponin I Rapid Test
BIOCREDIT COVID-19 IgG+IgM Duo