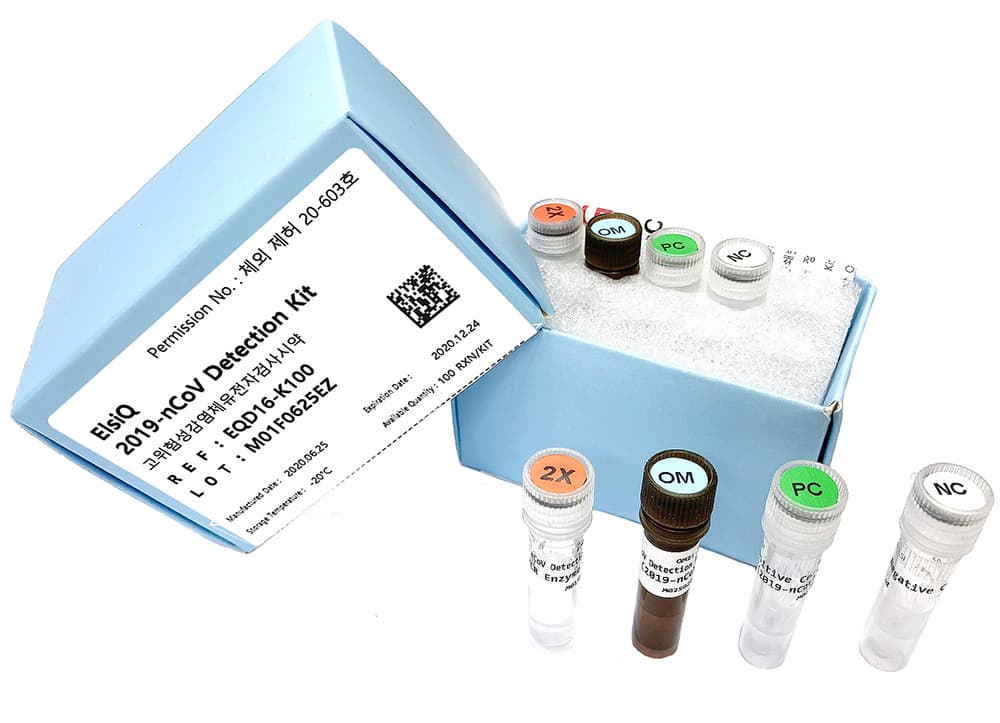
ElsiQ 2019-nCoV Detection Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, real-time pcr, covid-19, molecular diagnosis
- Category
- Medical Test Kit
20150410.png)
eONE Life Science Institute
- Verified Certificate
-
5



| Product name | ElsiQ 2019-nCoV Detection Kit | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | corona virus , real-time pcr , covid-19 , molecular diagnosis | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
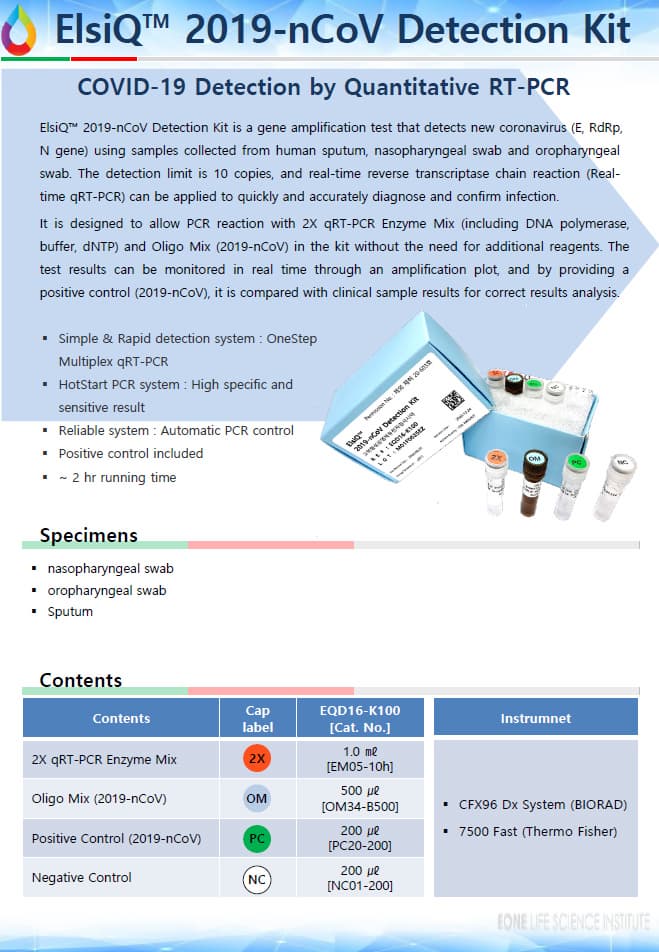
ElsiQ™ 2019-nCoV Detection Kit (Real-time RT-PCR)
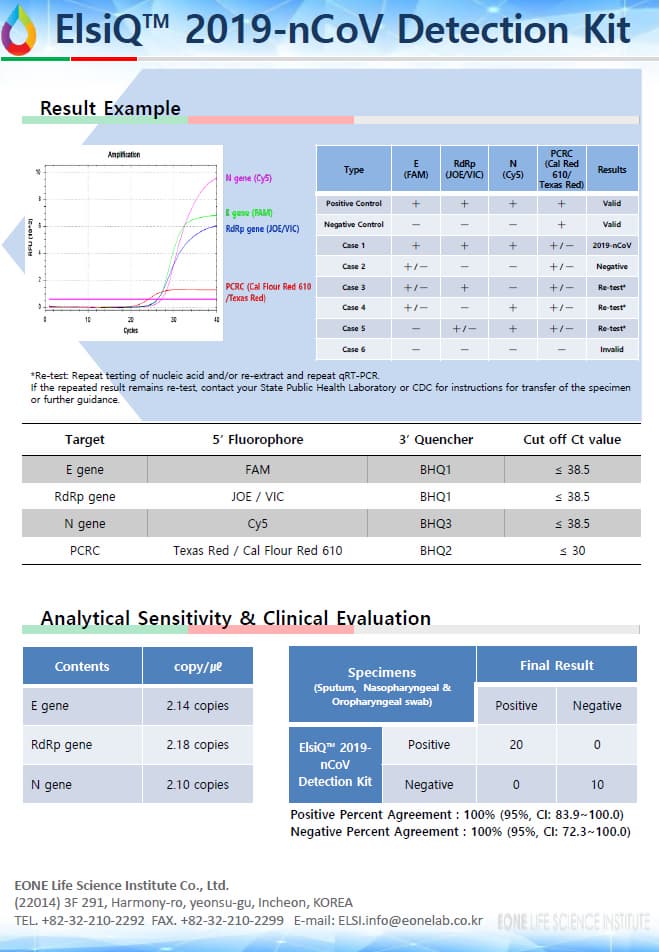
ElsiQ™ 2019-nCoV Detection Kit is a gene amplification test that detects new coronavirus
(E, RdRp, N gene) using samples collected from human sputum, nasopharyngeal swab and oropharyngeal swab. The detection limit is 10 copies, and real-time reverse transcriptase chain reaction
(Real-time qRT-PCR) can be applied to quickly and accurately diagnose and confirm infection.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
20150410.png)
- President
- Lee Chul Ok
- Address
- 291,Harmonyro, Yeonsu-gu, Incheon, Korea
- Product Category
- Health,Medical Test Kit
- Year Established
- 2010
- Company introduction
-

- Main Markets
-
 South Korea
South Korea
- Main Product
Related Products
Contour Test strips, Accu chek, One Touch, On Call Plus

R-ligo

Self-Stik Urine Strips
BioTracer FOB Rapid Test
AFP/PSA/CEA Rapid Test