KBE
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- orthodontic, dental equioment
- Category
- Dental Equipment

BioMaterials Korea Inc.
- Verified Certificate
-
17



| Product name | KBE | Certification | - |
|---|---|---|---|
| Category | Dental Equipment | Ingredients | - |
| Keyword | orthodontic , dental equioment | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information


Visiting Scholar, Department of Biochemistry,
University of Pennsylvania
Adjunct Professor, Department of Orthodontics,
University of Pennsylvania, Temple University
Visiting Scholar Division of Plastic Surgery,
the Children’s Hospital of Philadelphia
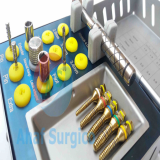
Kee’s Bone Expander Component

1. MARPE (Laboratory-fabricated)
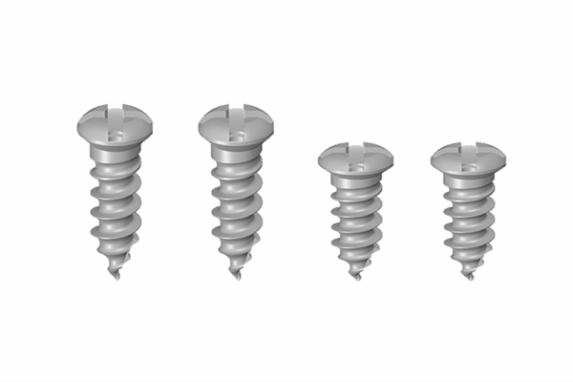
2. Bone screws (diameter 2.0 x 4EA)
(Anteriors : 9mm x 2, Posteriors : 7mm x 2)
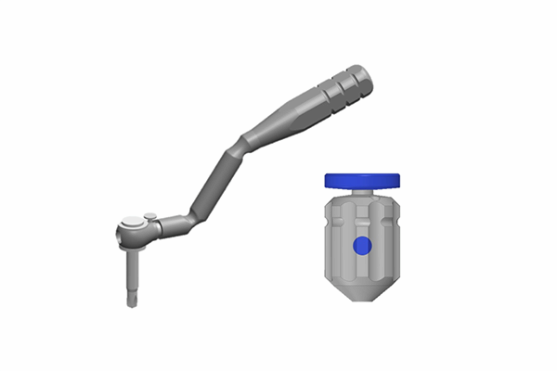
3. Contra angle Screwdriver
-Ratchet wrenches
-Mini Handle Driver

4. Screwdriver tips
-Short shaft
-Long shaft
Kee’s Bone Expander Guide
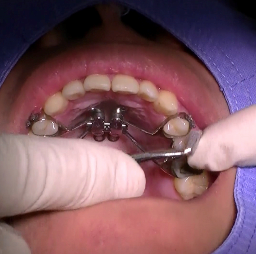
① 2-1. Kee’s bone expander Place/Position for Installation
First of all, space 1~2 mm/one to two milli metre apart from mid palatal soft tissue, and install the Kee’s bone expander stick to the mid palate maximally.
※ In case the space is larger, it is easy to fail since the fixation screw would be bended or sloped.
※ Supporting arm functions as a guide to insert the screw accurately, so it does not matter to skeletal expansion, even if the supporting arm moves a little.

② 1. Kee’s bone expander Insertion of Fixation Screw
1-1. To fix Kee’s bone expander, insert the second and third Bone screw in a diagonal direction about 2/3. / 2/3 diagonally.
1-2. After pre-fixing bone screws to fix for no.2 and 3, almost perpendicularly insert each screw in order.
※ When inserting the screw, bury the screw in the bone enough. (To minimize irritation for patients and expand easily)
1-3. Use 9mm surgical bone screw on the anterior side, and use 7 mm surgical bone screw on the posterior side.
1-4. On the expander no.1 and 2, insert the expander for/aim at the space between teeth no.1 and 2. Insert the expander no.3 and 4 near to the mid line of palatal.
1-5. When inserting bone screw, you had better use a hand driver (by hand) rather than instruments like engine etc.
※ If the fixation screw does not turn any more, discontinue inserting the screw to avoid odontoclasis/fracture.
Insert the screw by hand, or it would be difficult to recognize the above situation by instruments like engine.
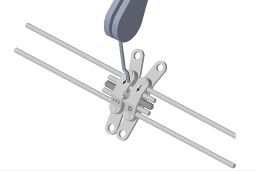
③ 3. pin
3-1. Decide/Set/Order the number of using pin upon consideration of patient’s arch form and bone density.
Turn the pin once a day in standard.
※ Four times Turn = One round (One turn -> 0.2mm movement)
3-2. Wait for the pin in case the pin does not be turned initially.
If diastema does not happen due to ankylosis after 2~3weeks, wait about 1~2 weeks more.
※ Take enough time to expand successfully.

④ 2-2. Kee’s bone expander for infants/children(the model) without supporting arm)
Application : children > adults / No impression / Rapid setting / Bonding simultaneous
※ Patients with RPE need to visit hospital about 4 times, but Kee’s bone expander can be installed within the day.
※ After adapting Kee’s bone expander for infants/children, fix with wax on the part of body base plate, and insert screw.
※ After finishing installation/installation, it is fine to turn the pin directly.
※ RPE requires 6 months totally, but Kee’s bone expander for infants/children is completed for 4 months.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Keun-Taek Oh
- Address
- #1013, Kolon Science Valley II, 55 Digital-ro, 34-gil, Guro-gu, Seoul, South Korea
- Product Category
- Other Dental Supplies
- Company introduction
-
BioMaterials Korea, Inc. is a leading manufacturer of precision orthodontic products and osteosynthesis(OMF, CMF, Plastic, and Neuro surgical bone screw, plate and instrument) products. BioMaterials Korea, Inc. has its own R&D center which strives to make not just high tech products, but also high quality products. Company products meet the internationl standard certificates (CE 0120, US FDA, KFDA, ISO 9001, and ISO 13485) in order to provide excellent quality products to our customers, and we strive for 100% customer satisfaction.
- Main Markets
-
 India
India
 Japan
Japan
 Malaysia
Malaysia
 South Korea
South Korea
 Spain
Spain
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Factory Information
-
BioMaterials Korea Inc.
- Main Product
Related Products
Bone Expander Kit Price

Dental Instruments Manufacturer Sialkot Pakistan

OSTEOTOMES

5 Axis Dental Lab CAD CAM Milling Machine Unit

DENCLE ANDANTE FUNCTIONAL TOOTHBRUSH_10 Pieces




































