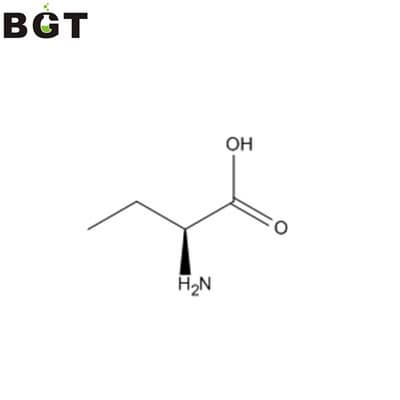
L-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Brand name
- BGT
- Payment Terms
- L/C,Others,T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- aminobutyric acid, l-2-aminobutyric acid, 1492-24-6
- Category
- Pharmaceutical Intermediates
Banff Green Technologies, Inc.
- Verified Certificate
-
8

| Product name | L-2-Aminobutyric acid, CAS 1492-24-6, Homoalaine | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | aminobutyric acid , l-2-aminobutyric acid , 1492-24-6 | Unit Size | - |
| Brand name | BGT | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
What is L-2-Aminobutyric acid?
In biochemistry, L-2-Aminobutyric acid is also known as homoalanine. It is a non-proteinogenic alpha amino acid. The straight two carbon side chain is one carbon longer than alanine, hence the prefix homo-.
L-2-Aminobutyric acid is an isomer of the amino acid aminobutyric acid. It has two other isomers, gamma-aminobutyric acid (GABA) and beta-aminobutyric acid. In contrast, GABA is a neurotransmitter and not used in protein synthesis, and it is almost always referred to as GABA.
Application of L-2-Aminobutyric acid
L-2-Aminobutyric acid is an intermediate for medicine.
Synthesis of L-2-Aminobutyric acid
Homoalaine is biosynthesised by transaminating oxobutyrate, a metabolite in isoleucine biosynthesis. It is used by nonribosomal peptide synthases. One example of a nonribosomal peptide containing homoalanine is ophthalmic acid, which was first isolated from calf lens.
Ways to synthetize L-2-Aminobutyric acid
1. With a transamination reaction from l-threonine and l-aspartic acid as substrates in a whole cell biotransformation using recombinant Escherichia coli K12.
The cells contained the cloned genes tyrB, ilvA and alsS which respectively encode tyrosine aminotransferase of E. coli, threonine deaminase of E. coli and α-acetolactate synthase of B. subtilis 168. The 2-aminobutyric acid was produced by the action of the aminotransferase on 2-ketobutyrate and l-aspartate. The 2-ketobutyrate is generated in situ from l-threonine by the action of the deaminase, and the pyruvate by-product is eliminated by the acetolactate synthase. The concerted action of the three enzymes offers significant yield and purity advantages over the process using the transaminase alone with an eight to tenfold increase in the ratio of product to the major impurity.
2. From 2-oxobutyric acid and benzylamine with an enantiomeric excess higher than 99%.
Asymmetric synthesis of an unnatural amino acid was demonstrated by (t)-transaminasefrom Vibrio fluvialis JS17. The reaction showed severe product inhibition by benzaldehyde ,which was overcome by employing a biphasic reaction system to remove the inhibitory product from the aqueous phase. In a typical biphasic reaction (50 mM2-oxobutyric acid,70 mM benzylamine and 2.64 U/ml purified enzyme)using hexane as an extractant ,conversion of 2-oxobutyric acid reached 96% in 5h whereas only 39% conversion was obtained without the product extraction.
3. From DL-2-aminobutyric acid and L-dihydroxysuccinic acid or D-dihydroxysuccinic acid.
With DL-2-aminobutyric acid and L-dihydroxysuccinic acid or D-dihydroxysuccinic acid comprising reacting in inorganic acid. After cooling and refrigeration, separate out the solid compound and recrystalize it with water. After the aminolysis reaction of the recrystalized compound with ammonia solution, L-2-Aminobutyric acid is gained through the process of cooling, filtration and cleanse.
L-2-Aminobutyric acid prepared by this way is with high purity, high yield and much lower cost. It is feasible for large scale industrial production.
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,Others,T/T | Shipping time | Negotiable |
- President
- Xue Yang
- Address
- Floor 29, CapitaMalls Plaza, No. 6088 Humin Rd., Minhang Dist.
- Product Category
- Antibiotic and Antimicrobial Agents,Inorganic Salts,Organic Intermediate,Pharmaceutical Intermediates
- Year Established
- 2014
- No. of Total Employees
- 101-500
- Company introduction
-
Banff Green Technologies, Inc. is a technologically innovative company that focuses on chemicals, materials, pharmaceuticals, crop science and environmental technology. The company headquarter is based in Shanghai, China. Our research and development team is comprised of a talented and experienced team of PhD’s with strong scientific backgrounds dedicated to our mission. We are also supported by strong infrastructure, which include state of the art lab facilities, two pilot facilities and seven manufacturing sites located in five provinces in China.Our core businesses span over the following areas: R & D, production and sales of fine and specialty chemicals, contract manufacturing services and custom synthesis.Our main products are: fluorinated fine chemicals (mainly Diethyl fluoromalonate and dimethyl fluoromalonate), D-p-Methyl Sulfone Phenyl Ethyl Serinate, Florfenicol, D-(-)-threo-2-Amino-1-(4-nitrophenyl)-1,3-propanediol, and chloramphenicol).Our corporate mission is to achieve environmental protection and sustainability while being socially responsible through our research and innovation. We sincerely hope to better serve our customers through every facets of their needs and together we can achieve a more sustainable future.
- Main Product
Related Products
Hydrocoralliaire- Klonopin- Lexapro
Betamethasone
Betamethasone 17-valerate
Cas 62595-74-8

Water Soluble Chitosan


































 China
China



