HbA1C Analyzer (Clover A1c)
The CLOVER A1c® Plus Analyzer is an IVD(In Vitro Diagnostic Device) device for measuring Hemoglobin A1c
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- analyzer, blood glucose, hba1c

OSANG HEALTHCARE CO., Ltd
- Verified Certificate
-
16





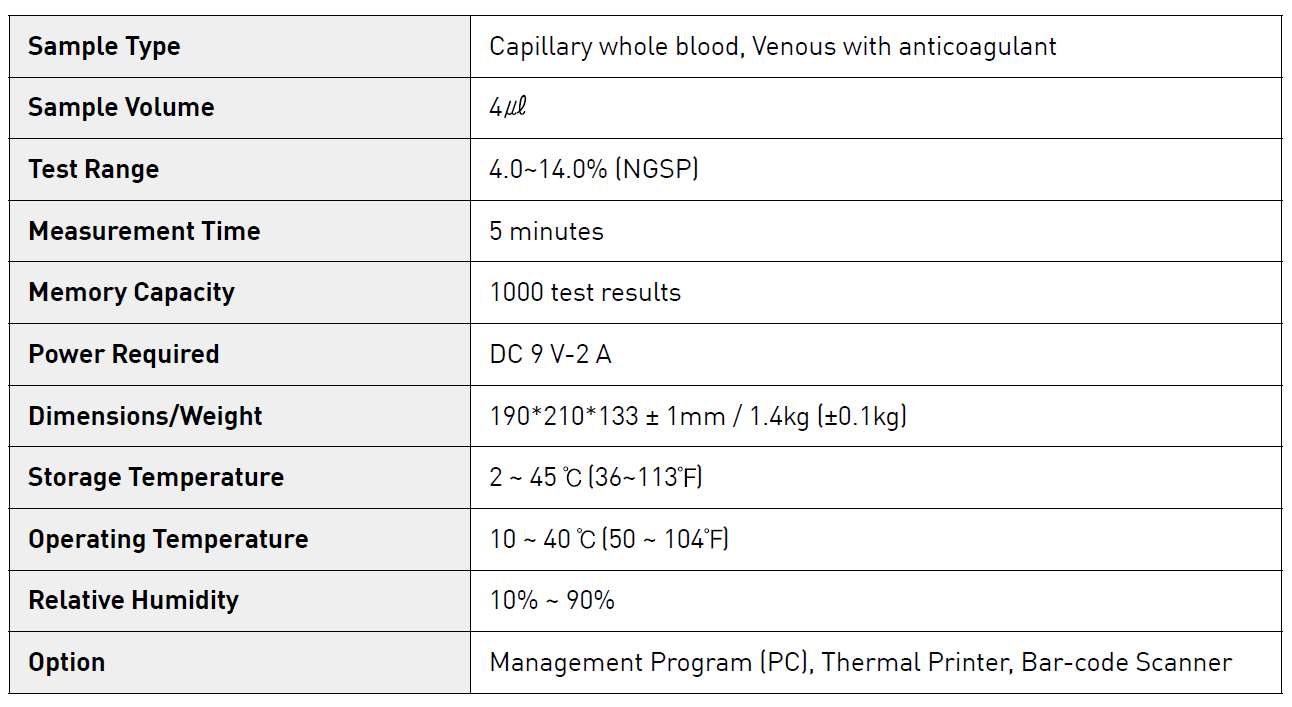
| Product name | HbA1C Analyzer (Clover A1c) | Certification | - |
|---|---|---|---|
| Category | Other Examination & Testing Instrumnet | Ingredients | - |
| Keyword | analyzer , blood glucose , hba1c | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 5000 |
| Supply type | ODM,OEM | HS code | 9018199000 |
Product Information
HbA1C Analyzer (under research)
- The analyzer provides quantitative measurement of the hemoglobin A1c(%HbA1c) levels using a fingerstick blood sample.
- This can help reduction the risk of diabetes complications
- HbA1c levels is measured small sized monitor, designed for homecare system, fast, easily, accurately
Specifications
- Principle : Boronate affinity binding precipitation /Electrochemical reaction
- Method : Spectrophotometry/ Amperometry
- Test Range : 3~13 % / 0~1,000 mg/gl
- Measuring time : 5 min (HbA1c) / 5 sec (Glucose)
- Operating Temperature : room temperature(18-28 °C , 64-82 °F )
- Sample : whole blood, 10ul
Contents
- Meter
- HbA1c / glucose detection system
- Lancing device, Lancets, Pincette
- Calibration cartridge
- Manual, Quick ref.
- Adapter (no AA size battery) - Cartridge
- HbA1c cartridge
- Dilution solution, Capillary tube
- Manual
1. Principles of Operation
The reagent solution contains reagent that lyse erthrocytes and boronate bead that binds cisdiols
of glycated hemoglobin. Inserting the Reagent Pack into the Cartridge is instantly lysed
releasing the Hemoglobin and the boronate bead binding the glycated hemoglobin. firstly, The
blood sample mixture is rotated to the measurement zone of the cartridge, where the amount
of total hemoglobin in the blood sample is measured by the photo sensor. next, The washing
solution washes out non-glycated Hemoglobin from the blood sample, thus the amount of
glycated hemoglobin can be measured. Finally, The ratio of glycated hemoglobin with respect
to total Hemoglobin in the blood sample is calculated.
2. Contents

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

OSANG HEALTHCARE CO., Ltd
-
16





- President
- SeunEuk Hong
- Address
- 132 Anyangcheondong-ro, Dongan-Gu, Dongan-gu, Anyang-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit
- Year Established
- 1996
- No. of Total Employees
- 101-500
- Company introduction
-
OSANG Healthcare has been a leader in the Korean IVD (In-Vitro Diagnosis) industry since its founding in 1996, ever expanding a wide array of medical devices for biochemical, immunoassay, molecular diagnostics, and digital healthcare.
OSANG Healthcare Co., was the first South Korean SARS CoV 2 test kit producer to receive authorization from the U.S. Food & Drug Administration in April 2020.
The company has supplied countries all over the world and has already sold 760,000 tests to US customers, including the Federal Emergency Management Agency, Agency (FEMA). Giving the first priority to our customers and partners, we will further develop powerful synergies with other affiliates in the group.
- Main Markets
-
 China
China
 Germany
Germany
 Greece
Greece
 India
India
 Indonesia
Indonesia
 Iran
Iran
 Italy
Italy
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
 Yemen
Yemen
- Factory Information
-
Osang Healthcare
- Main Product
- Attached File
Related Products
HURO PATH LBC(Liquid based Cytology) System

OsteoPro (Ultrasound Bone Densitometer)

Medical Headlamp

Nurugo CPR manikin
Osteopro (X-ray Bone densitometer, DEXA)


































_2.png)
_2.png)
_3.png)
_4.png)
_5.png)
_6.png)
 South Korea
South Korea



