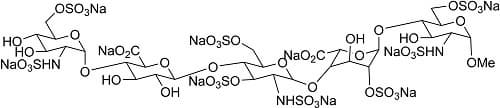
Fondaparinux Sodium
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- active pharma ingredient, api, fondaparinux
- Category
- Organic Intermediate
Tianjin Chase Sun Pharmaceutical Co., Ltd
- Verified Certificate
-
10

| Product name | Fondaparinux Sodium | Certification | - |
|---|---|---|---|
| Category | Organic Intermediate | Ingredients | - |
| Keyword | active pharma ingredient , api , fondaparinux | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
Title: Fondaparinux Sodium
CAS Registry Number: 114870-03-0
CAS Name: Methyl O-2-deoxy-6-O-sulfo-2-(sulfoamino)-a-D-glucopyranosyl-(1®4)-O-b-D-glucopyranuronosyl-(1®4)-O-2-deoxy-3,6-di-O-sulfo-2-(sulfoamino)-a-D-glucopyranosyl-(1®4)-O-2-O-sulfo-a-L-idopyranuronosyl(1®4)-2-deoxy-2-(sulfoamino)-a-D-glucopyranoside 6-(hydrogen sulfate) decasodium salt
Additional Names: fondaparin sodium; SR-90107A; Org-31540
Molecular Formula: C31H43N3Na10O49S8
Molecular Weight: 1728.08
Percent Composition: C 21.55%, H 2.51%, N 2.43%, Na 13.30%, O 45.37%, S 14.84%
Properties: White powder after lyophilisation. [a]D23 +48° (c = 0.61 in water).
Optical Rotation: [a]D23 +48° (c = 0.61 in water)
Therap-Cat: Antithrombotic.
Keywords: Antithrombotic.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Mr. Yao
- Product Category
- Antibiotic and Antimicrobial Agents,Blood System Agents,Cardiovascular Agents,Organic Intermediate,Pharmaceutical Intermediates
- Year Established
- 1996
- No. of Total Employees
- 1001-2000
- Company introduction
-
Tianjin Chase Sun Pharmaceutical Co. Ltd was founded in 1996 and the joint-stock transformation was completed in 2000. In 2009, Chase Sun pharmaceutical was listed on the Shenzhen Stock Exchange and became the first group of national GEM listed companies, also called as the first of Tianjin. (Stock code: 300026)
In 2013, the company won top 100 comprehensive list of listing Corporation, the best venture growing company in Gold Bull Award 2013, the most investment value listed Company, top 20 of the most competitive pharmaceutical listed Company in China, and the top 10 management team award of 2013 Chinese GEM Listing Corporation.
Products: Xuebijing Injection (Modern Traditonal Chinese Medicine), Fasudil hydrochloride oral preparation; and anti-tumor, immunoregulation, Cardiovascular and cerebrovascular, Anaesthesia API and intermediates;
Technology transfer center of Chase Sun Pharma covers an area of 25,000 square meters with a total investment of 300 million Yuan. Technology transfer center possesses production lines for synthetic drugs, such a polusaccharide, peptides, anti-cancer drugs and production lines for large volume injection, small volume injection, freeze-dried powder and oral solid preparation. Each line is constructed in accordance with the GMP Standard (2010 revision) and has a laboratory for comprehensive testing and analysis with a complete production quality control system. Technology transfer center is committed to providing the customers services of process development of APIs and the intermediates from pre-clinical to the industrialization.
Services (Product means API, intermediate and fine chemical)
1: Own product production, sales; (GMP & Non GMP);
2: Product Contract Manufacturing; (GMP & Non GMP);
3: Product research and development, achievements transformation including pilot studies and scale-up trials for new preparation and new products;
4: Studies of controlling the conditions and process of production;
5: Validation of the maturity of the technical;
6: Providing drugs for clinical trials and small batch production;
7: Technology research, pilot project and technology programs advising and services;
8: Drug registration advising and services;
- Main Product
Related Products

Methomyl
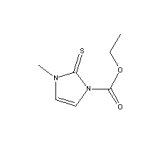
Carbimazole, CAS NO.: 22232-54-8

3-chloro-4-fluroaniline (CAS 367-21-5)
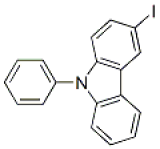
3-Iodo-N-phenylcarbazole, CAS NO.: 502161-03-7

M-Terphenyl


































 China
China



