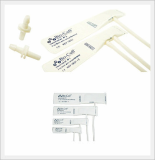
BMT Reusable NIBP Cuff
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Surgical Glove
Bio Medical Technologies Co., Ltd.
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
17



| Product name | BMT Reusable NIBP Cuff | Certification | - |
|---|---|---|---|
| Category | Surgical Glove | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Bio-Cuff
Bio-Cuffs are engineered to meet current clinical guidelines for proper cuff sizing and fit set forth by AAMI. Manufactured without a separate bladder-eliminating the need to remove, clean, or replace a bladder. Bio-Cuff is remarkably easy to clean and both cuff and tubing is 100% latex free for eliminating the concerns of people with latex sensitivities.
Compatible monitor models
Nellcor, Criticare, Datascope, SpaceLabs, Philips, BCI, Drager, Fukuda-Densh, Nihon-Kohden, Critikon ,Welch Allyn, Marquette, GE, Datex-Ohmeda, Siemens etc.
Specification
| SIZE | Limb Circumference |
Single tube | Double tube | Qty/pack |
| Thigh | 38-50cm | 1101 | 1101-2 | 5 |
| Big Adult | 31-40cm | 1201 | 1201-2 | 5 |
| Adult | 23-33cm | 1301 | 1301-2 | 5 |
| Child | 18-26cm | 1401 | 1401-2 | 5 |
| Pediatric | 12-19cm | 1501 | 1501-2 | 5 |
| Infant | 8-13cm | 1601 | 1601-2 | 5 |
| Connector Feature |  |
 |
 |
 |
 |
 |
| Style(BMT REF) | B | F | M | S | BH | IH |
- Choose the appropriate connector you need and add at the part number ex) 1301-B, 1201-2-IH
- Prevent from the contamination of all risk
- Soft-touch material for patient comfort
- Compatible with most of monitors' NIBP module
- Available with single and double tube
- Wide range of sizes
- Latex free
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Shin Joo Ho
- Address
- 431-17, Sindang-dong, Jung-gu, SEOUL, 100-450, KOREA
- Product Category
- Medical Devices
- Company introduction
-
Bio Medical Technologies(BMT) is first pioneer of pulse oximetry probes in Korea to replace high cost original probes in 2000. Now BMT exports over 80 country customers all over the world and BMT products are awarded many public, governmental, global tenders as our qualities are verified for 10years history. BMT probes are certified ISO 9001,13485,9019 , CE marked and KGMP. In 2009, BMT acquired 510k(FDA) approval to penetrate U.S market where almost original sensors actually comes from. We'd like to introduce our company and product with great confidence of quality. It's your time to choose prime quality product with good price solution.
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Portugal
Portugal
 South Africa
South Africa
 Spain
Spain
- Main Product