Ceftriaxone Sodium
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Pharmaceuticals

Kyongbo Pharmaceutical Co., Ltd.
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
17



| Product name | Ceftriaxone Sodium | Certification | - |
|---|---|---|---|
| Category | Pharmaceuticals | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
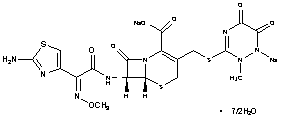
CAS No. : [104376-79-6]
Formula: C18H16N8Na2O7S3 . 3½H2O
Mol. Weight: 661.61
Conformed to: USP, EP, BP, JP, KP
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Kang, Tae Won
- Address
- 174 Silok-ro, Asan-Si, Chungcheongnam-Do, 336-020, Korea
- Product Category
- Other Pharmaceutical Chemicals
- No. of Total Employees
- 101-500
- Company introduction
-
ISO 9001(QMS) / 14001(EMS), GMP Certification
Kyongbo Pharmaceuical Co., Ltd. is a subsidiary company of Chong Kun Dang pharmaceutical corp. The company is a pioneer in the active pharmaceutical industry in Korea, founded in 1987, with a future-oriented outlook for fine chemistry as a promising high-tech industry for the 21st century and with the humanitarian mission to contribute to the life and health of mankind.
With its primary goal set in developing new pharmaceutical substances, the company operates under the main strategy the secure superior competitive edge by simplifying and streamlining its manufacturing procedures through technological innovation and producing highly profitable raw materials for drugs.
- Main Markets
-
 China
China
 Italy
Italy
 Japan
Japan
 South Korea
South Korea
- Main Product
Related Products

DL-ALANINE

Jeju Sasa Extract
Asco-1 Ascorbic Acid 1000mg
_2.jpg)
Sentrip Oral Dissolving Film 10mg/20mg(TADALAFIL 20mg)

Korean Herbal Health Drink, Wilfordi Root