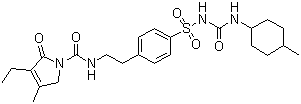
Glimepiride(CAS No. 93479-97-1)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- pharmaceutical ingredient, cas no. 93479-97-1, diabetes treatment, intermediate cas no.119018-29-0.
- Category
- Organic Intermediate , Pharmaceutical Intermediates
Weihai Dizhiya Pharmaceutical Co., Ltd.
- Country / Year Established
-
 China
/
China
/
- Business type
- Others
- Verified Certificate
-
13

| Product name | Glimepiride(CAS No. 93479-97-1) | Certification | - |
|---|---|---|---|
| Category |
Organic Intermediate
Pharmaceutical Intermediates |
Ingredients | - |
| Keyword | pharmaceutical ingredient , cas no. 93479-97-1 , diabetes treatment , intermediate cas no.119018-29-0. | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Glimepiride, API, CAS No. 93479-97-1, Assay: >99.5%,
Its intermediate with CAS No. 119018-29-0 is available.
Pharmaceutical use,
Lowest price and best quality from CHINA.
GMP certified manufacturing.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
Weihai Dizhiya Pharmaceutical Co., Ltd.
- Country / Year Established
-
 China
/
China
/
- Business type
- Others
-
13

- President
- Mr Li
- Address
- 55 Qilu Avenue, Eco. & Tech. Development Zone, Weihai, Shandong, CHINA 264205
- Product Category
- Organic Intermediate,Pharmaceutical Intermediates
- Company introduction
-
Weihai Disu Pharmaceutical Co. Ltd.,is a subsidiary company of Disha Pharmaceutical Group which is among Top 100 pharmaceutical corporations in China. As a high-tech enterprise with joint venture, Weihai Disu Pharmaceutical Co., Ltd. was founded in 2005 and involved in R&D, manufacture and marketing of APIs and Intermediates.
Ⅰ.Our quality control system:
Sound quality control and assurance systems ensure the whole manufacture operation and quality control comply with GMP requirements.
Ⅱ.Our R&D system:
There is a professional R&D team of high educational background talents, led by experienced senior engineers, which ensures a continued supply of new products.
Also we have been cooperating with Peking University and Fudan University on some new product research.
Ⅲ.Our production capacity:
With 6 GMP certified manufacturing plants and other modern facilities, we have production capacity of 200 tons per year and more than 100 products are produced.
Ⅳ.Our products:
We always adhere to “Authentic Disha Product, Honest Disha”. Our product could reach kinds of Pharmacopoeia standards: CP, BP, USP and EP. It is of high quality and also affordable to customers all over the world.
Look forward to cooperating with you.
- Main Markets
-
 Bangladesh
Bangladesh
 Egypt
Egypt
 India
India
 Indonesia
Indonesia
 Japan
Japan
 Pakistan
Pakistan
 South Korea
South Korea
 Thailand
Thailand
 Viet Nam
Viet Nam
- Main Product
Related Products

120/80 SYMBIOSAL _ antihypertensive salt
Drug, medicine, API, pharmaceutical raw material, surgical dressing

Water Soluble Chitosan
Phenylbutazone

Eriocitrin