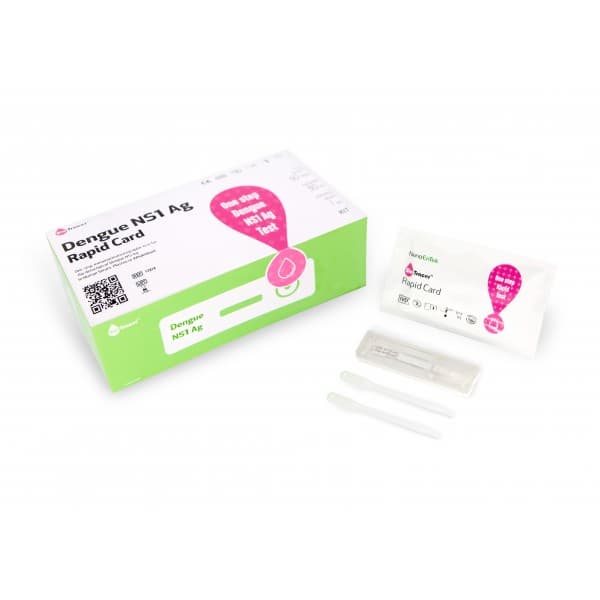
Dengue Combo Rapid Card
Detect NS1 antigen and IgG/IgM antibodies
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others
- Production method
- OBM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Devices
NanoEnTek Inc.
- Recent Visit
- Jan 13, 2025
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
17



| Product name | Dengue Combo Rapid Card | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Material | - |
| Keyword | dengue rapid test , in vitro diagnostic rapid test , poct | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 0 |
| Supply type | OBM | HS code | - |
Product Information
- Fast test time (15~20minutes) & easy procedure (3 steps)
KEY ADVANTAGES
Excellent Convenience & Performance
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product
Related Products

PRF BOX Implant

Modular type ENT workstation_XU5 Visual

Deesse Mask, LED Mask
Bone Expander Kit Price

Dental Implant Ratchet Hex Drivers