Total Solution for COVID-19
NanoEntek provides a total solution for the detection and surveillance of COVID-19 infection. En-swer™ COVID-19 RT-PCR Kit detects the presence of SARS-CoV-2 virus itself through real-time reverse-transcription polymerase chain reaction. FREND™ COVID-19 IgG/IgM Duo is a point-of-care testing (POCT) which can be used to check whether patients have developed immune responses to SARS-Cov-2 using human plasma.
Choose the right COVID-19 test based on your needs.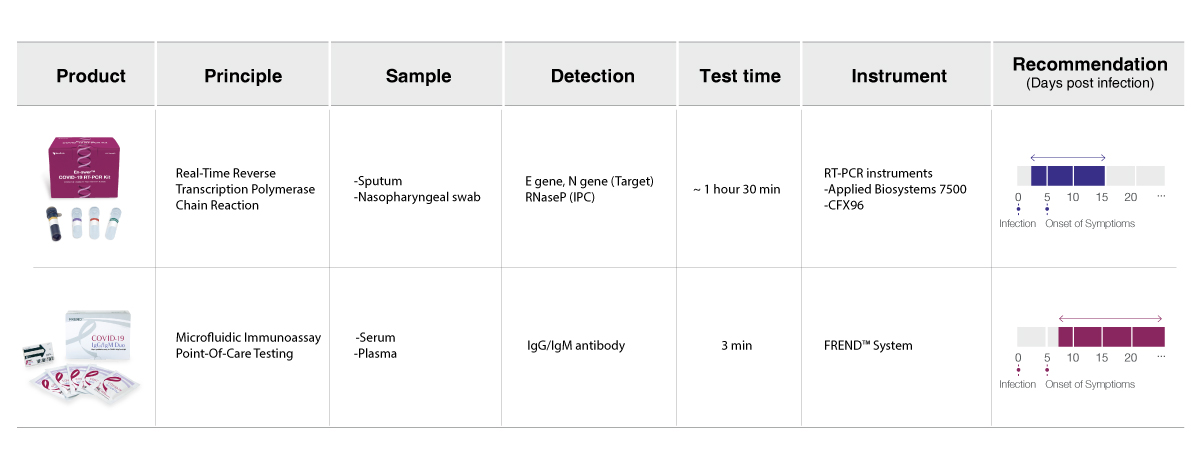
Microfluidic Qualitative ImmunoassayFREND™ COVID-19 IgG/IgM Duo
The FREND™ COVID-19 IgG/IgM Duo is a fluorescence immunoassay (FIA) for use with the FREND™ System. It is designed for the qualitative detection of the nucleocapsid protein of SARS-CoV-2 directly from nasopharyngeal swab specimens directly from individuals suspected with COVID-19 by their healthcare provider.

Instructions for useAccurate result in 3 minutes!
With just simple steps of operation, the FREND™ system supports quick decision-making. 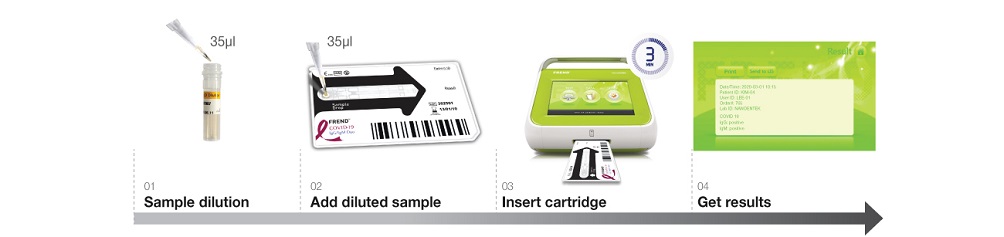
Clinical result
Clinical Performance Evaluation
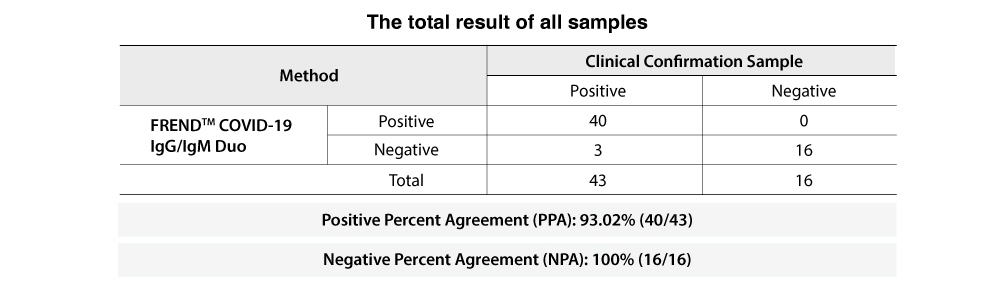
The total of 59 clinical specimens (43 positive and 16 negative) confirmed with RT-PCR were tested with the FREND™ COVID-19 IgG/IgM Duo.
It shows 93% PPA (Positive Percent Agreement) and 100% NPA (Negative Percent Agreement) as shown below.
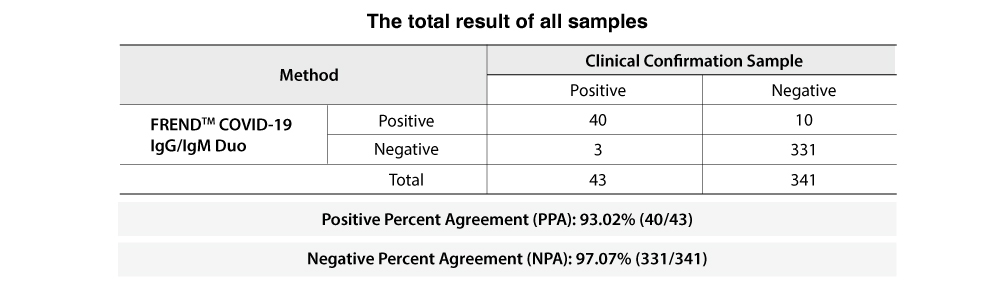
The following table includes 325 negative specimens collected before the outbreak of COVID-19.
It shows 93% PPA (Positive Percent Agreement) and 97% NPA (Negative Percent Agreement) as shown below.
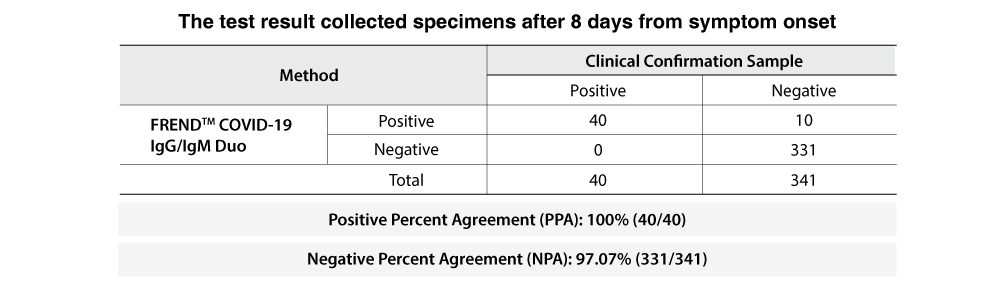
For specimens collected after day 8 from the onset of symptoms, the FREND™ COVID-19 IgG/IgM Duo shows 100% PPA.
Therefore, it is recommended to test specimens at a minimum of day 8. A similar tendency was found in another cohort study⑴.
1. The sensitivity of the FREND™ COVID-19 IgG/IgM Duo early after infection is unknown.
2. It is suggested from a study that the rate of seropositivity for IgM was lower than IgG after 14 days or longer after symptom onset⑴.

Clinical result
Performance characteristics
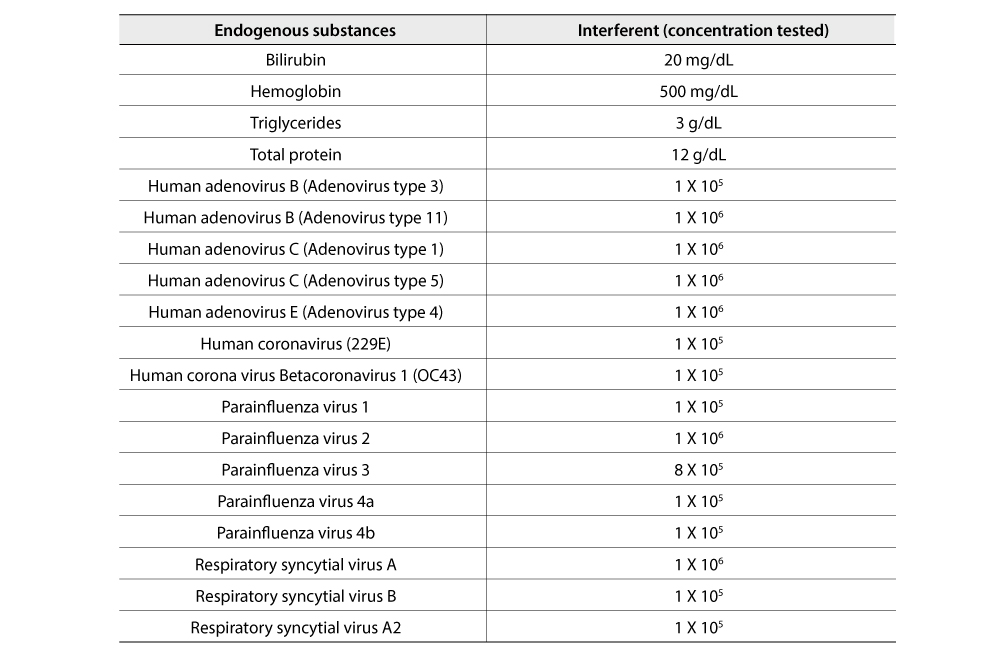
Interference
In the FREND ™ COVID-19 IgG/IgM Duo test, it was confirmed that the following interferences were not affected.
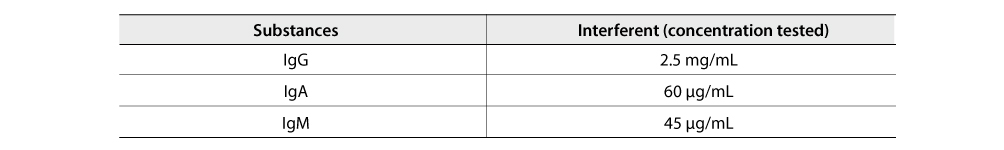
Class specificity
The following substances were evaluated for potential cross-reactivity with the FREND™ COVID-19 IgG/IgM Duo. No significant cross-reactivity was found itself.
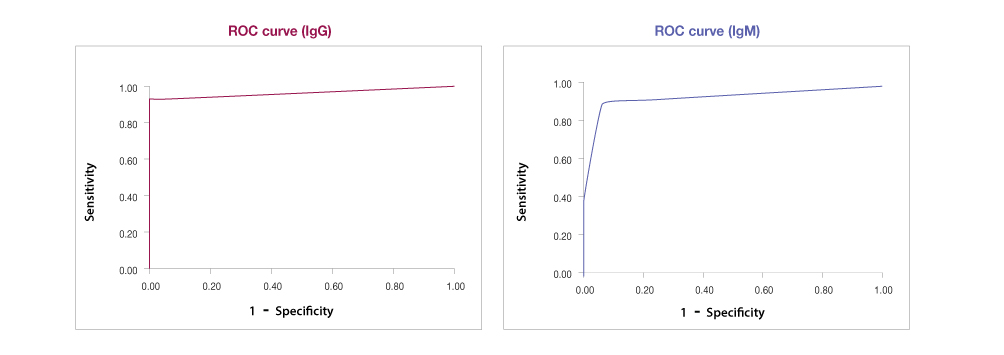
ROC curve
Receiver Operating Characteristic
The FREND™ COVID-19 IgG/IgM Duo provides results qualitatively, but the principle of detection is quantitative.
Therefore, Cut-Off-Index (COI) is determined quantitatively by testing the specimens collected after day 8 from the onset of symptoms using the FREND™ System.
The FREND™ System shows the test result as negative if the Cut-Off-Index (COI) is < 1.0
If the Cut-Off-Index (COI) is ≥ 1.0, then the test result will be provided as positive. The results are shown below.
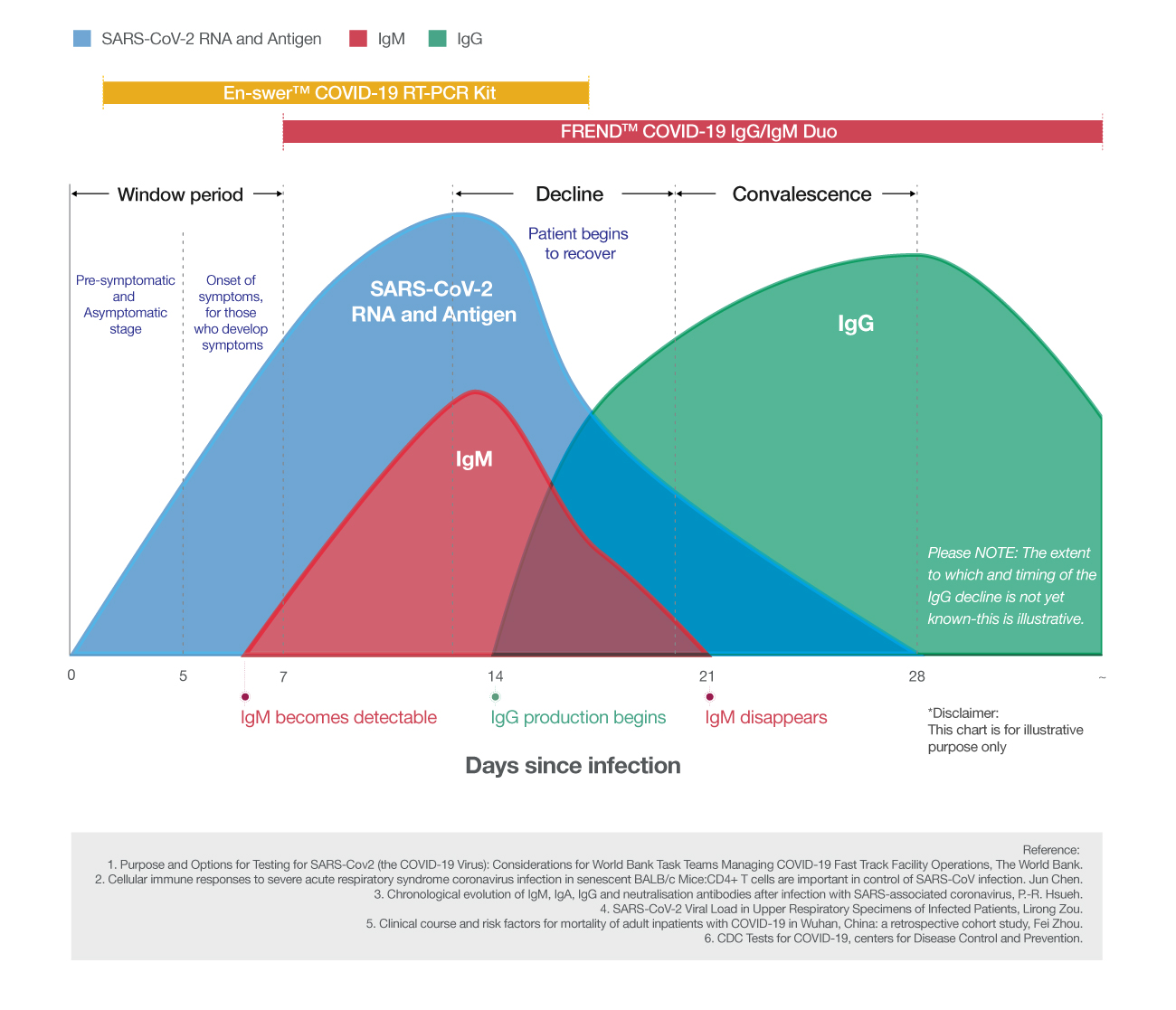
Background information
Why serological test is necessary?
Molecular diagnostic kit such as RT-PCR is very useful in detecting the presence of viral genes. However, this cannot tell whether the person has developed an immune response to SARS-CoV-2. Therefore, serological test using blood samples is required to detect whether symptoms developed from infection or the infection was asymptomatic (Centers for Disease Control and Prevention is also developing its own serological test using IgG and IgM)⑴.

The following study suggests the importance of serological test.
After the onset of symptoms, the sensitivity of positive PCR result showed higher than 90% from day 1 to day 3. However, it decreased below 80% and 50% on day 6 and day 14, respectively (Figure 1). The combination of PCR with serological test increased the positive sensitivity by 47% (Figure 2)⑵.
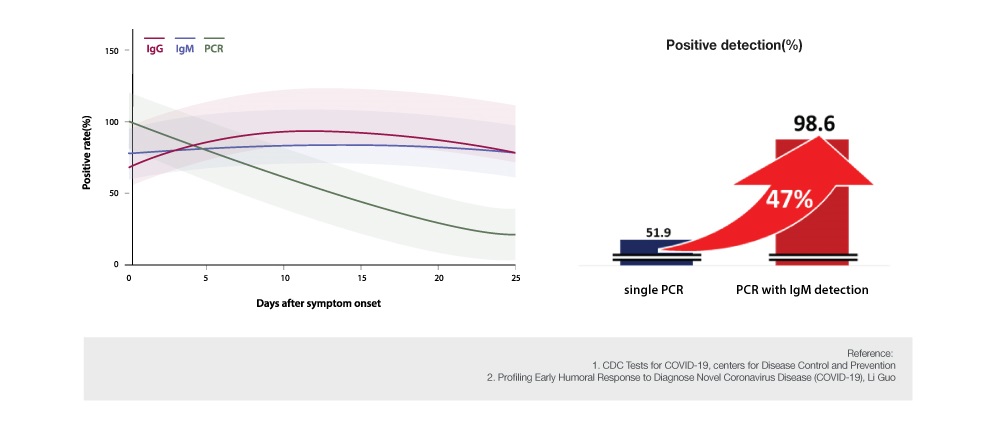
*208 plasma samples (82 confirmed patients & 58 unconfirmed patients who tested negative)
Suggestion from Studies
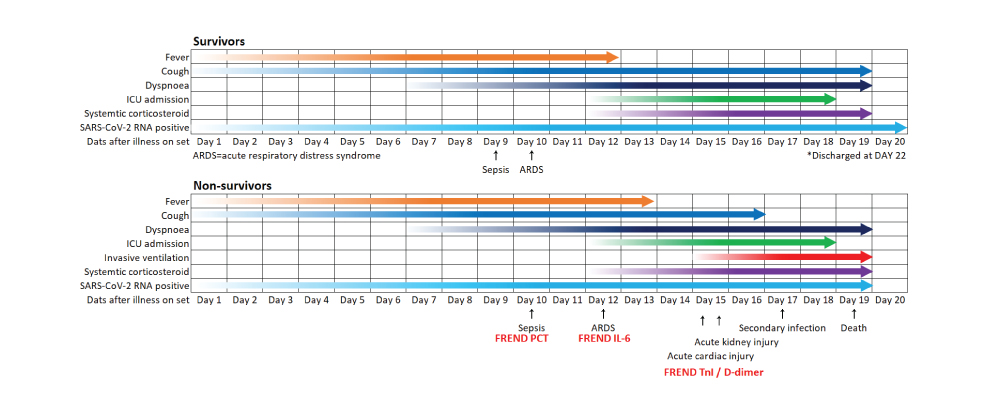
Using FREND™ System for Extension Study (Quantitative)
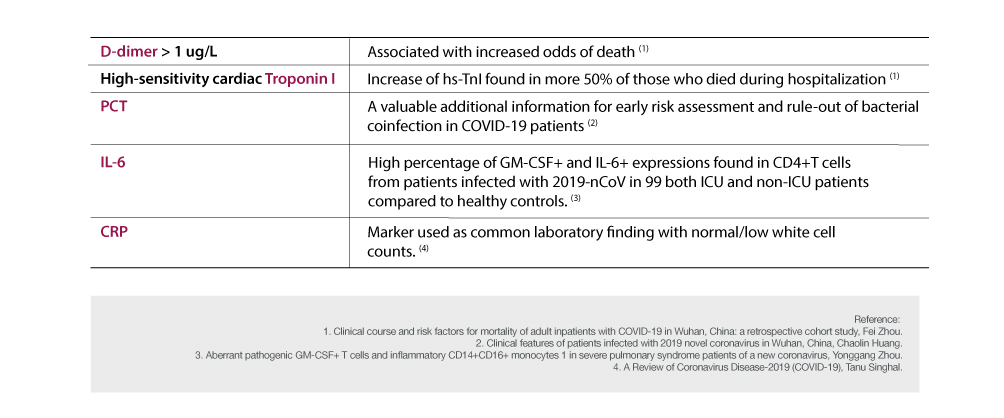
Most of the rapid kits only offer test on Serological Diagnostic Kits using IgG and IgM. However, the FREND™ System can also be used to manage and assess COVID-19 patient in secondary infection or comorbidities that could follow using the additional markers, D-dimer, Troponin I, PCT, IL-6, and CRP (C-reactive protein) through quantitative detection.
FREND™ System items
Total 14 Quantitative items available
The FREND™ System provides a variety of other quantitative items, which can be tested for a total of 14 different items.
Effective Data Management
Laboratory Information System Connectivity
In the event of a pandemic, the management of a vast amount of clinical result is important. However, many laboratories face the challenge in arranging the essential information effectively(1).
The FREND™ System which is LIS compatible provide following features:
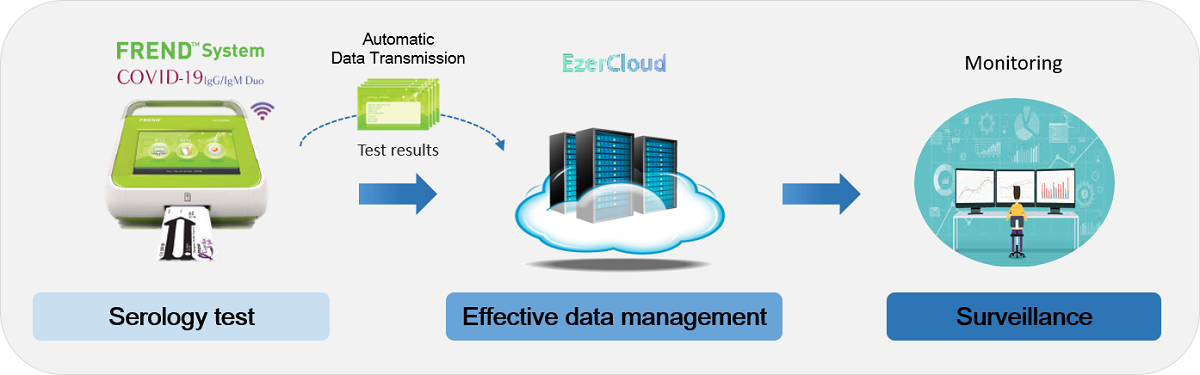
From Diagnosis to Surveillance
Global Smart Surveillance System for Infectious Disease
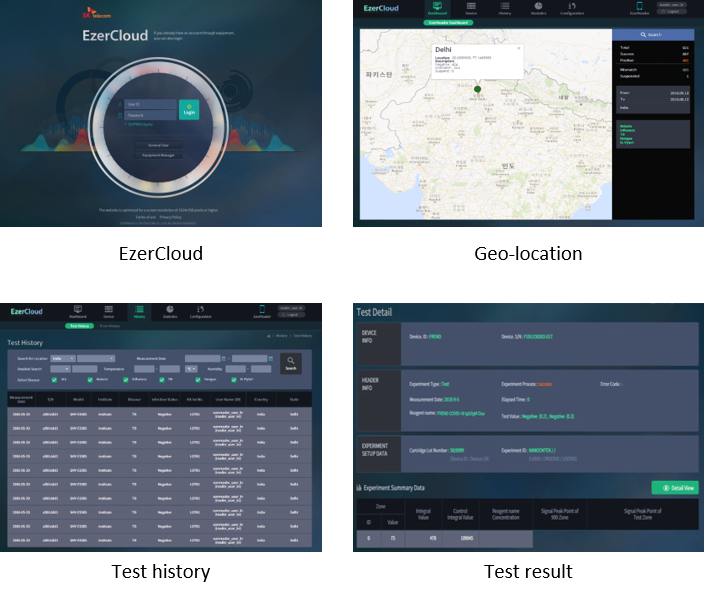
With the ‘FREND System’ and ‘EzerCloud’, the COVID-19 surveillance is available from diagnosis to monitoring. The ‘EzerCloud’ store the data using the serology test results collected from ‘FREND System’ with cloud connectivity and inform the result to an authorized person or designated organization in case of target disease emergence.
The ‘EzerCloud’ system is a solution for Infectious Disease Surveillance that provides the data of COVID-19 cases (number of cases and each positive/negative results) and tracks the outbreak. It can monitor the intensity, geographic spread and severity of COVID-19 in the population in order to estimate the burden of disease. It is also helpful for understanding the actual infection rate and how it is spreading across the world.
Notification
• This test has not been reviewed by the FDA.
• Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
• Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
• Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
• Not for the screening of donated blood.
 South Korea
/
2000
South Korea
/
2000












 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom