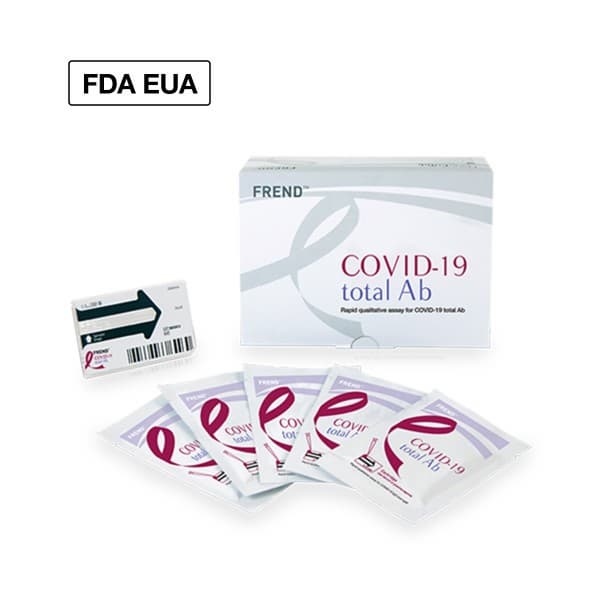
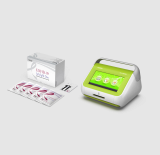
FREND COVID-19 total Ab
Qualitative test for COVID-19 total Ab
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others
- Production method
- OBM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- in vitro diagnostic, poct, in vitro diagnostic test, covid 19
- Category
- Medical Devices
NanoEnTek Inc.
- Recent Visit
- Jan 13, 2025
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
17



| Product name | FREND COVID-19 total Ab | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Material | - |
| Keyword | in vitro diagnostic , poct , in vitro diagnostic test , covid 19 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 0 |
| Supply type | OBM | HS code | - |
Product Information
- U.S. FDA EUA approved
- 3 minutes | Fast result
- 2 steps | Easy to use
- 96.7% & 98.8% | Positive & Negative Percent Agreement
- Microfluidic Qualitative Immunoassay
- LIS connectivity (data management)
The FREND™ COVID-19 total Ab is a fluorescence immunoassay (FIA) which can be used to check whether patient has developed immune response to SARS-CoV-2 using human plasma. For COVID-19 total Ab, its detection is based on a fluorescent immunoassay showing qualitative result.
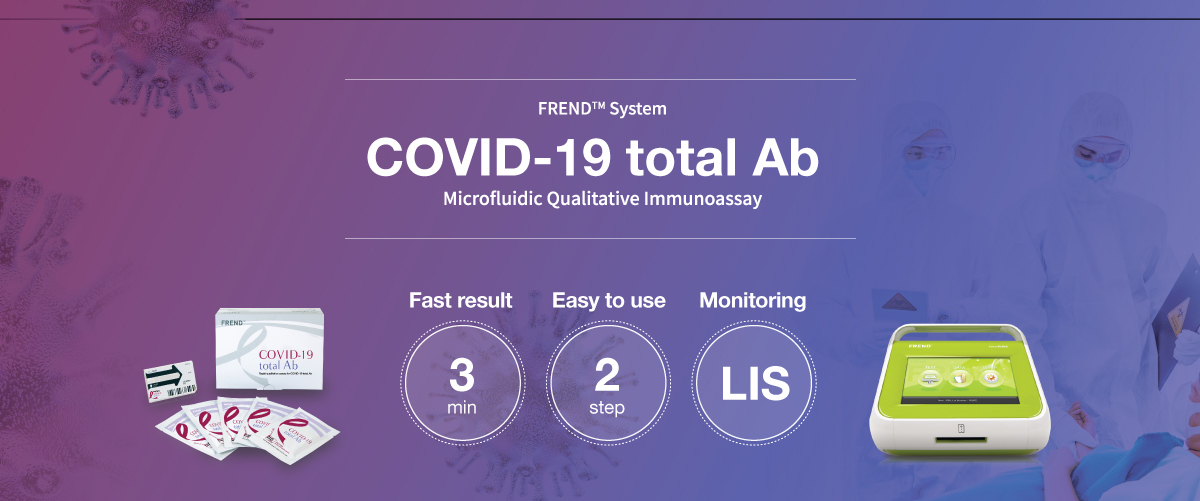
With just simple steps of operation, the FREND™ system supports quick decision-making.

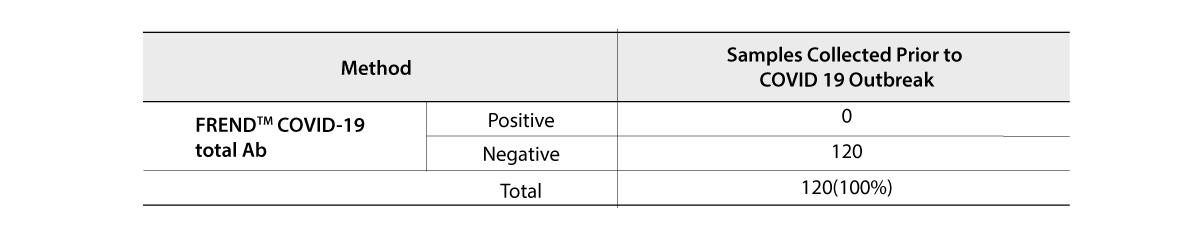
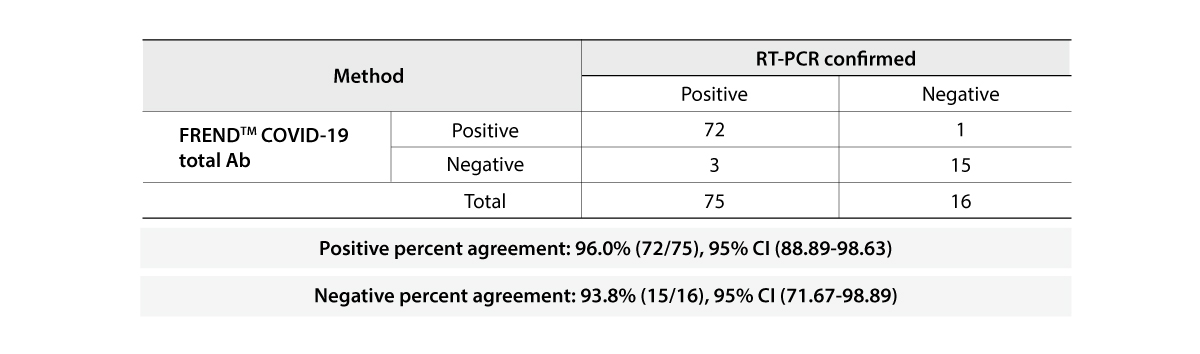
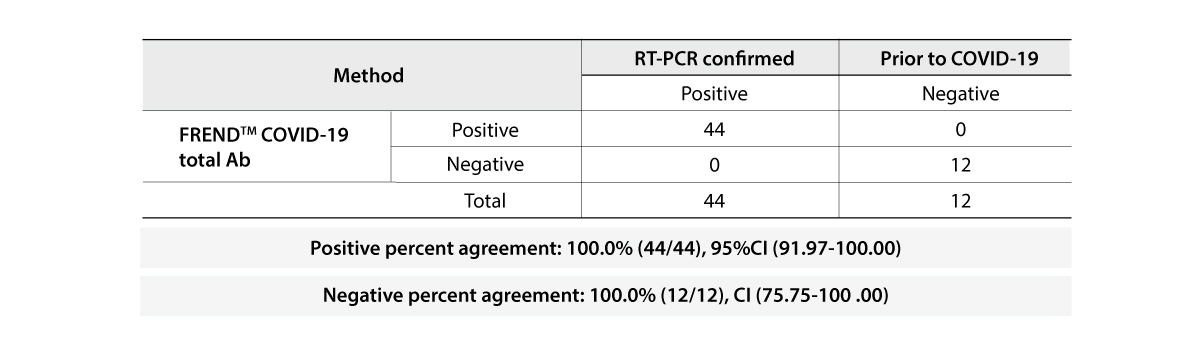
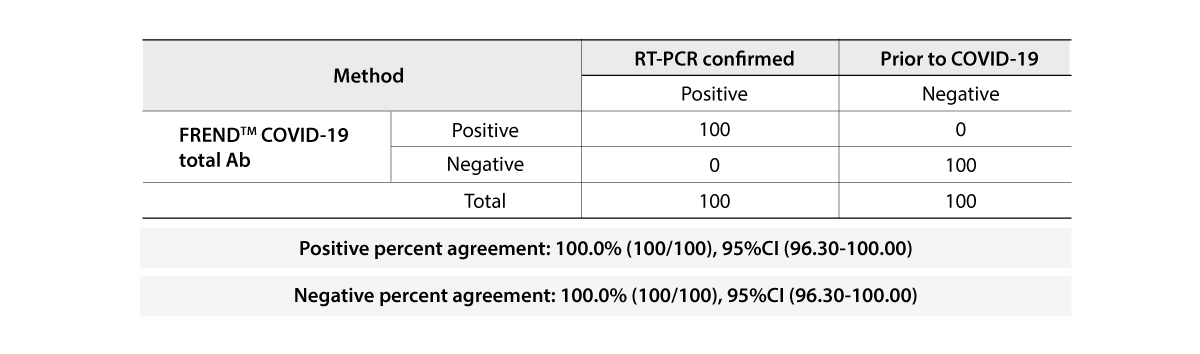
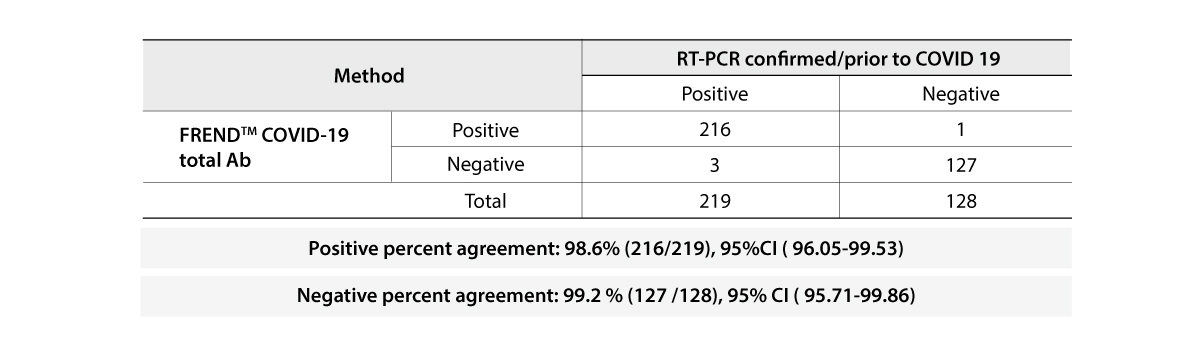
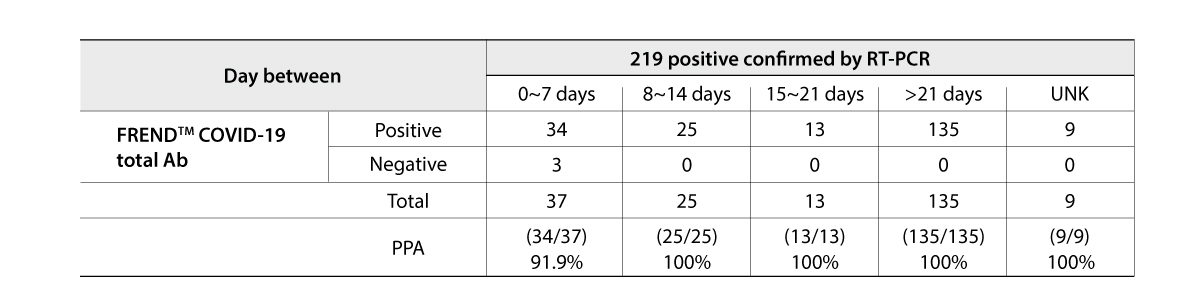
The FREND COVID-19 total Ab from NanoEntek was tested on August 19, 2020 at the Frederick National Laboratory for Cancer Research (FNLCR) sponsored by the National Cancer Institute (NCI).


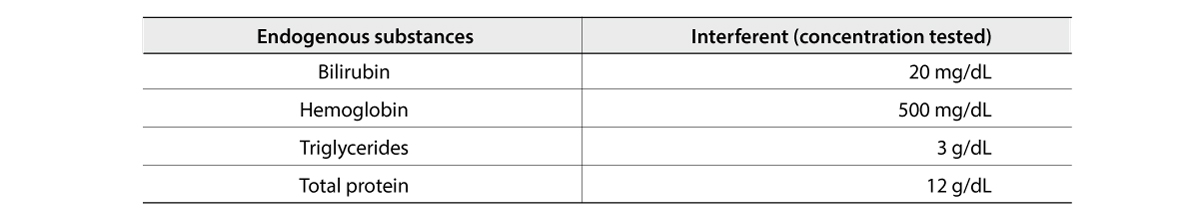
Effective Data Management
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product