LEXYAL CE FILLER, Facial Care, Dermal Filler, Fillers, Korean Filler, Antiwrinkle,Certificate Europe
LEXYAL HYALURONIC ACID / DERMAL FILLER LIDOCAINE / 1SYRINGE
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- LEXYAL
- Payment Terms
- MoneyGram,Others,T/T,Western Union
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- dermal filler, skin care, hafiller, plastic surgery filler

Double J Holdings Co., Ltd.
- Membership
- PRO
- Recent Visit
- Jan 08, 2025
- Country / Year Established
-
 South Korea
/
2022
South Korea
/
2022
- Business type
- Manufacturer
- Verified Certificate
-
2


| Product name | LEXYAL CE FILLER, Facial Care, Dermal Filler, Fillers, Korean Filler, Antiwrinkle,Certificate Europe | Certification | - |
|---|---|---|---|
| Category |
Other Beauty Products
Facial Care Medical Consumables Other Facial Care Other Medical Consumables |
Ingredients | HA,HA FILLER,HYALURONANIC ACID,HYALURONIC ACID FILLER,LIDOCAINE |
| Keyword | dermal filler , skin care , hafiller , plastic surgery filler | Unit Size | - |
| Brand name | LEXYAL | Unit Weigh | 0 mg |
| origin | South Korea | Stock | 10000 |
| Supply type | Available | HS code | 3304999000 |
Product Information

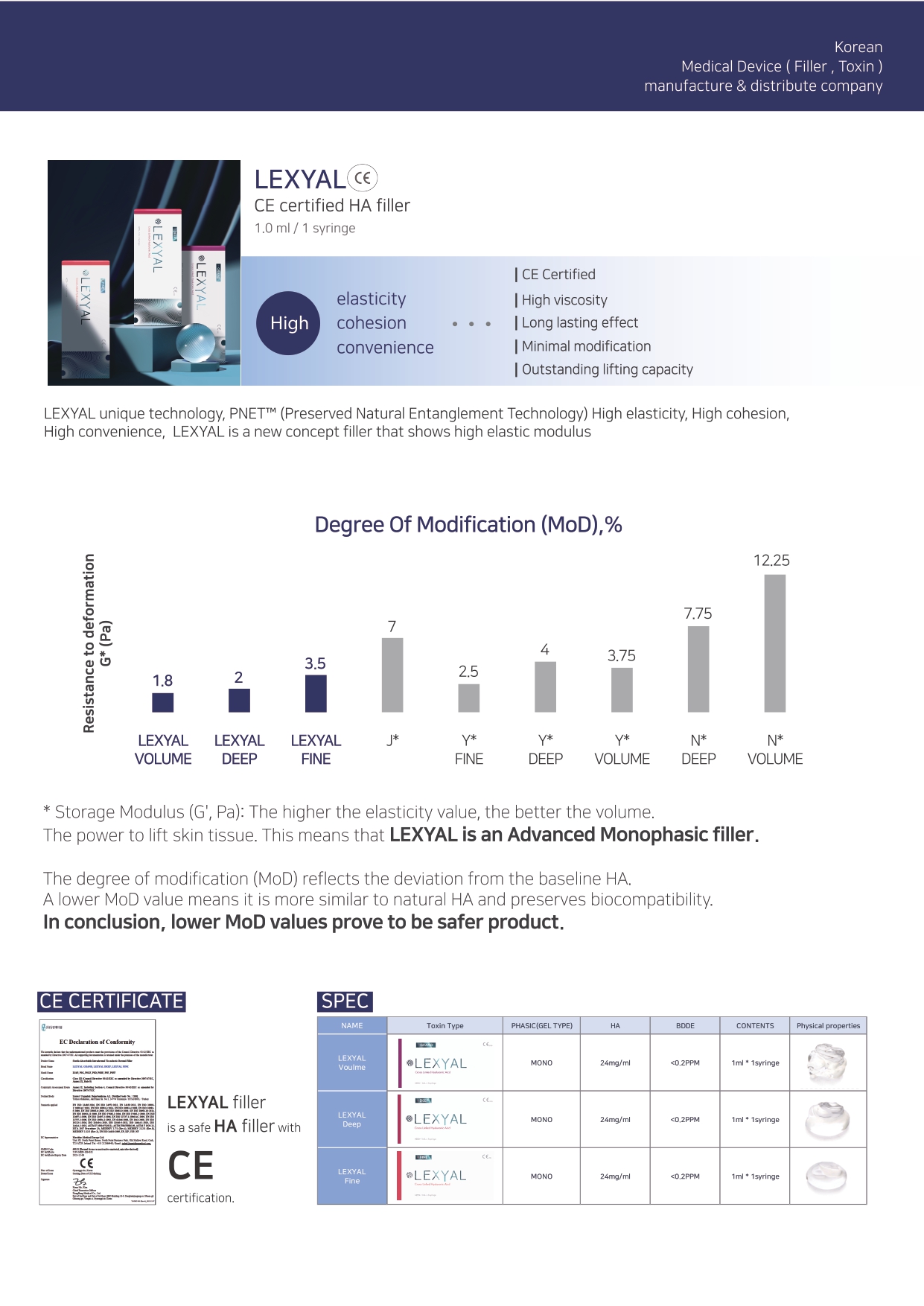
Sterile Absorbable Intradermal Viscoelastic Dermal Filler Cross-linked Hyaluronic Acid
Sterile Absorbable Intradermal Viscoelastic Dermal Filler (LEXYAL) is a transparent, viscous, biodegradable and stabilized cross-linked hyaluronic acid which is obtained by bacterial fermentation.
LEXYAL is supplied in a disposable glass syringe to be able to inject into deep dermis.
The content of the syringe is sterilized using moist heat. The syringe is equipped with a plunger
stopper, finger grip and plunger rod. The syringe is packed in a blister together with the hypodermic needle sterilized using ethylene oxide.A pre-clinical evaluation, in vivo comparative assessment of HA(Hyaluronic acid) dermal filler, at Chung-Ang University for one year showed that the product remains implanted in human skin tissues at least for 6 months.
And, it is considered that the product will be completely biodegradable in human body at the end of 1 year of implantation.
Composition
Cross-linked hyaluronic acid gel (24 mg/mL)
One syringe contains 1.0 mL
Distributor
Double J Holdings
2F, 271, Digital-ro, Guro-gu, Seoul, Republic of Korea
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | MoneyGram,Others,T/T,Western Union | Shipping time | Negotiable |

- President
- PARKJONGRYUL
- Address
- gurodong 212-13,2F, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2022
- No. of Total Employees
- 1-50
- Company introduction
-
This is ' Double J Holdings
' Manufacture dermal filler,skin Booster and provide various Korean medical devices such as dermal fillers, lipolysis, skin boosters, botulinums.
web : medicaljj.net
https://wa.me/message/VQJWSOKT2W4ZP1
- Main Markets
-
 Iran
Iran
 Iraq
Iraq
 Oman
Oman
 U.A.E.
U.A.E.
 U.S.A
U.S.A
- Main Product
- Attached File
Related Products

Ovaco B.P Cell Ampoule

Korean Hot Selling Natural OKA Facial Mask Sheet Collection
_2.jpg)
SOQU Rose Line (Mist, Cream, Serum)

eyelash essence
Dr. JART+ DERMASK Water Jet Vital Hydra Solution







































