GENEDIA W Dengue NS1 & lgM/lgG Combo
Dengue NS1 & lgM/lgG Test
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available,OBM,ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable

GC Medical Science CORP.
- Recent Visit
- Jan 20, 2025
- Country / Year Established
-
 South Korea
/
2003
South Korea
/
2003
- Business type
- Manufacturer
- Verified Certificate
-
17


| Product name | GENEDIA W Dengue NS1 & lgM/lgG Combo | Certification | - |
|---|---|---|---|
| Category |
Other Examination & Testing Instrumnet
Laryngoscope Other Monitoring & Diagnostic Equipment |
Material | - |
| Keyword | dengue , ns1 , rapid kit , lgm/lgg | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 200 |
| Supply type | Available,OBM,ODM,OEM | HS code | - |
Product Information
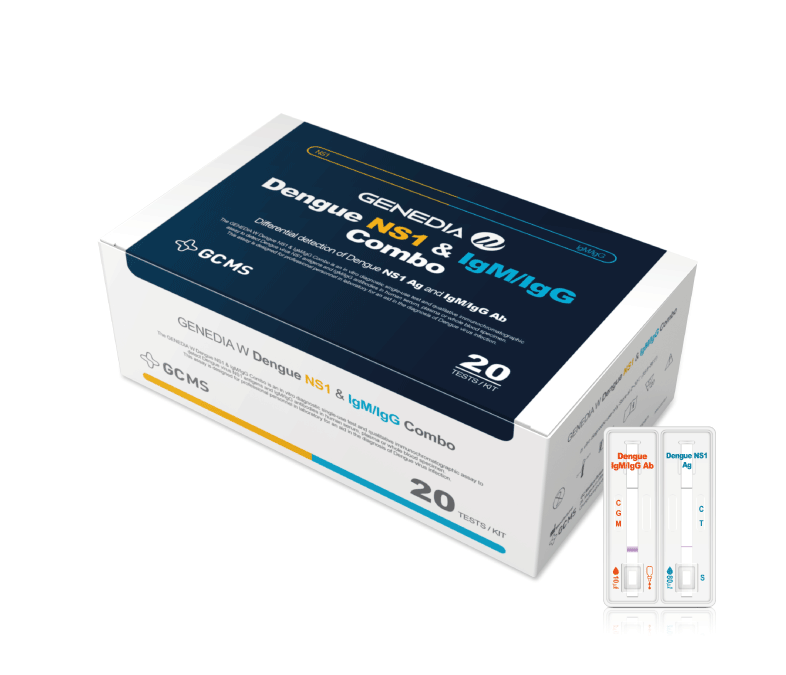
The GENEDIA W Dengue NS1 & IgM/IgG Combo test is an in vitro diagnostic single-use test and qualitative immunochromatographic assay to detect Dengue virus NS1 antigens and IgM/IgG antibodies in human serum, plasma or whole blood specimen. This assay is designed for professional personnel in laboratory for an aid in the diagnosis of Dengue virus infection.
This dual-target approach allows for a comprehensive assessment of Dengue virus infection by identifying both early and later stages of the disease.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

GC Medical Science CORP.
- Country / Year Established
-
 South Korea
/
2003
South Korea
/
2003
- Recent Visit
- Jan 20, 2025
- Business type
- Manufacturer
-
17


- President
- Seung-Sam Seo
- Address
- Yongin-si, Guseong-eup, Bojeong-ri,#303, Giheung-gu, Yongin-si, Gyeonggi-do, Korea
- Product Category
- Glucose Meter,Medical Test Kit,Multi-Parameter Monitor,Other Health Care Products,Other Monitoring & Diagnostic Equipment
- Year Established
- 2003
- No. of Total Employees
- 101-500
- Company introduction
-
We started as a blood type diagnosis business of Green Cross in 1972 and established it in 2003 based on technology and know-how in the field of medical devices and diagnostic reagents. The business currently in operation consists of diagnostic reagents, hemodialysis fluids, and chronic disease projects.
We are a leading diagnostic company in future industries with global performance and quality systems with international certification such as CE as well as the Ministry of Food and Drug Safety in Korea. In particular, in the case of overseas businesses, we focus on field diagnostic products and export measuring devices and reagents such as glycated hemoglobin, blood sugar, and hemoglobin developed based on differentiated patent technology to more than 90 countries around the world.
- Main Product
- Attached File


































_2.jpg)
_2.jpg)
_2.jpg)