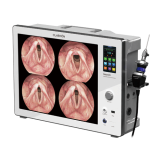
CELB ACE™ System
CELB ACE™ System is an in vitro diagnostic device [Class I]
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- L/C
- Production method
- ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable

K-BioCELF Inc.
- Verified Certificate
-
1

| Product name | CELB ACE™ System | Certification | - |
|---|---|---|---|
| Category | Other Monitoring & Diagnostic Equipment | Material | - |
| Keyword | liquid biopsy , in vitro diagnosis , nucleic acid extraction , circulating tumor cell | Unit Size | - |
| Brand name | - | Unit Weigh | 250 kg |
| origin | South Korea | Stock | 2 |
| Supply type | ODM,OEM | HS code | - |
Product Information
CELB ACE™ System : Cancer is the number one cause of death. It is estimated that 1 in 3 people develop cancer. The most important way to reduce cancer deaths is “early cancer screening.” However, invasive cancer diagnosis causes patient pain, concerns about complications, and is expensive. For early cancer diagnosis, it is important to extract cancer cell-derived nucleic acid (ctDNA) and circulating tumor cells (CTC), but separation is not easy due to PBMC and protein components in the blood. To overcome these difficulties, the CELB ACE™ System is a liquid biopsy in vitro diagnostic medical device that can extract ctDNA from the blood of cancer patients and isolate circulating cancer cells.
CELB ACE™ System is an in vitro diagnostic device [1], which can rapidly extract and purify ctDNA and CTC from liquid samples(plasma, serum and urine) and PBMC(peripheral blood mononuclear cell), respectively. Isolated high-quality ctDNA and CTC are suitable for PCR, ddPCR, Genotyping, NGS, and Immunostaining.
[Application]
• Early Cancer Diagnosis : ctDNA methylation with NGS
• Provides Evidence for Predicting Tumor Metastasis & Anti-Cancer Therapeutic Effects : CTC enumeration
• Personalized / Companion Diagnosis for Cancer Therapy : Cultivation of Isolated CTC
• Tumor Metastasis Research & New Drug Development : Single Cell Analysis of CTC
Performance | Specification |
Sample Range | •24
Well Plate : up to 3000㎕ |
Magnetic Rod | •24
Well Plate : 4 x 6 |
Size | •W1,800mm x D900mm x
H1,000mm |
Heater | •Controllable Channel : 1 •Controllable
Temp : Up
to
150℃ •Temp.
Sensor :
K Type Thermocouple |
Cooler | •Controllable
Channel : 2 •Controllable
Temp : Lower to 0℃ •Temp.
Sensor : K Type Thermocouple |
Screen | •Pixel : 1280 X 800 •Keyboard |
Sterilization Function | •UV C LED (280nm) •Continuous
Use Time : 1
hour |
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C | Shipping time | Negotiable |

K-BioCELF Inc.
-
1

- President
- Oh Myung Ryurl
- Address
- #503, 77, Changnyong-daero 256beon-gil, Yeongtong-gu, Suwon-si, Gyeonggi-do, Republic of Korea, Yeongtong-gu, Suwon-si, Gyeonggi-do, Korea
- Product Category
- Other Equipment,Other Monitoring & Diagnostic Equipment
- Year Established
- 2019
- Company introduction
-
K-BioCELF Inc., was established in March 2019, and a Bio Venture company whose main businesses is the developments of technology based new drugs, in vitro Diagnosis and Automated Bioprocess Robotic Systems based on core technology.
Major products include CELB ACE™ System(Liquid Biopsy System, IVD), CELB™ SEP-STD (Automated Blood Separation System), CELP™ (Artificial Skin Model Production System), CELF™ (Advanced Biopharmaceuticals Production System), CELH™ DDR-SP (Reagent Dispensing System), CELH™ DST (Automated Microbial Inoculation System), and Molecular genetic diagnostic kits, and so on.
Advanced biopharmaceuticals: stem cells, iPSC-derived stem cells, organoids, immune cells (NK, T cells, etc.), and CGT (cell and gene therapeutics; CAR-T, CAR-NK, CAR-Macrophage, etc.).
- Main Markets
-
 Canada
Canada
 India
India
 Indonesia
Indonesia
 Thailand
Thailand
 U.S.A
U.S.A
- Main Product
- Attached File


































 South Korea
South Korea