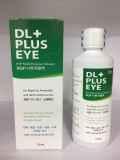
Eye Plus Multi Solution(Contactlens care solution)
There is no need to use a separate protein remover or daily cleaning agent
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Eye Plus Multi Solution
- Payment Terms
- T/T
- Production method
- ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Other Eyewear , Other Pharmaceuticals
| Product name | Eye Plus Multi Solution(Contactlens care solution) | Certification | HALAL , CE |
|---|---|---|---|
| Category |
Other Eyewear
Other Pharmaceuticals |
Ingredients | - |
| Keyword | contact lens multi - solution , contact lenes care , contact lens multipurpose solution | Unit Size | - |
| Brand name | Eye Plus Multi Solution | Unit Weigh | - |
| origin | South Korea | Stock | 20000 |
| Supply type | ODM,OEM | HS code | 3307903000 |
Product Information
Multi-Purpose Solution
HARD SOFT CONTACT LENS MULTIPURPOSE SOLUTION
Usage
Step 1: Drop 3 drops of this solution on both surfaces of the lens and rub for 20 seconds.
Step 2: Rinse the surface of the lens thoroughly with this solution to remove any residue remaining on the surface of the lens. Step 3: Put the cleaned lenses in the lens case and soak them in this clean solution for at least 4 hours. These lenses can be worn at any time. There is no need to separately rinse again with saline. If any residue is left on the lens, they can be used after rinsing again with this solution. The lens case cap should be closed if the lenses are not to be put on immediately. Since saline does not have a sterilizing effect, do not mix this solution with saline
Protein removal from contact lenses
When cleaning and disinfecting lenses daily with this solution, proteins are also removed, so there is no need to use a separate protein remover or daily cleaning agent
1. CE(MDD) certificate
2. Iso13485
3. Certificate of korea Halal
4. Certificate of product-specific approved exporter
5. Stand-alone test (Inoculum challenge test)
6. Antimicrobial preservative efficiency test
7. Agar diffusion test
8. Skin sensitization test
9. Ocular irritation test
- Product Info Attached File
- Verified Certificate
-


B2B Trade
| Price (FOB) | Negotiable | transportation | Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- OH heung-jun
- Address
- 31026, Seobuk-gu, Cheonan-si, Chungcheongnam-do, Korea
- Product Category
- Other Pharmaceuticals
- Year Established
- 2010
- No. of Total Employees
- 1-50
- Company introduction
-

Human Bio Co., Ltd. is a company that researches, develops, and manufactures only contact lens management solutions. We have various certificates such as CE(MDD) certificate, Iso13485, Certificate of korea Halal, certificate of product-specific approved exporter, and certificates of excellent technology enterprise companies. It is a manufacturer specializing in lens management solutions with abundant production and export experience.
- Main Markets
-
 Saudi Arabia
Saudi Arabia
- Main Product
- Attached File
Related Products

PPAEBAR Lactoferrin Dietary Supplement
_2.jpg)
VINIX ORAL DISSOLVING FILM 50mg,100mg(SILDENAFIL 50mg, 100mg)
Daehan New Pharm Vitamin C injection

VYCOZ ACETATE SUN-JELIN, SUNGLASSES, lightweight sunglasses, sports glasses, screwless sunglasses
__2.jpg)
Disposable Health Mask (KF94)


































_2.png)

_2.png)
 South Korea
South Korea


_2.png)