GBR_Allograft Bone
100% Cortical Allograft and mineralized FDBA (Freeze-Dried Bone Allograft)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Hans Biomed
- Payment Terms
- L/C,T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- allograft, bone graft, dental implant, gbr

Osstem Implant Co.,Ltd.
- Membership
- VIP
- Recent Visit
- Jan 07, 2025
- Country / Year Established
-
 South Korea
/
1997
South Korea
/
1997
- Business type
- Manufacturer
- Verified Certificate
-
3



| Product name | GBR_Allograft Bone | Certification | FDA |
|---|---|---|---|
| Category |
Dental Equipment
Dental Consumables Dental Handpiece & Accessories Other Dental Equipment Other Dental Supplies |
Material | ALLOGRAFT |
| Keyword | allograft , bone graft , dental implant , gbr | Unit Size | - |
| Brand name | Hans Biomed | Unit Weigh | - |
| origin | South Korea | Stock | 200 |
| Supply type | Available | HS code | 300190 |
Product Information
SureOss (Allograft Bone)
Freeze-dried SureOss without cortical bone demineralization!


SureOss is freeze-dried allogeneic bone that does not decalcify cortical bone and preserves growth factors and other protein and mineral factors that promote regeneration of damaged areas. Therefore, during bone grafting, bone conduction and osteoinduction help bone regeneration.
Its materials are secured through strict medical history and blood tests for tissue donors concerning the donor's medical history, social activity, microbiological and immunoserologic tests accroding to the US FDA regulations and the European Tissue Banking Association guidelines.
SureOss is supplied in two types: chip bone and powder bone. Although it is difficult to feel the difference in clinical results, because of the convenience of use, the two are used in combination for maxillary sinus bone grafting and the powder type is mainly used for GBR.
[ Feature ]
- FDBA (Freeze-Dried Bone Allograft)
- 100% Allograft cortical Bone
- Particle Size (Powder : 200~850㎛ / Chip : 850 ~ 1000㎛ )
- Manufacturer : Hans Biomed Corp.
- FDA Registration
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,T/T | Shipping time | Negotiable |

- President
- Haesung KIM
- Address
- 8F, 3, Magokjungang 12-ro, Gangseo-gu, 07789, Gangseo-gu, Seoul, Korea
- Product Category
- Dental Equipment,Dental Unit,Dental X-Ray Equipment,Other Dental Equipment,Other Dental Supplies
- Year Established
- 1997
- No. of Total Employees
- over 2000
- Company introduction
-
Implants power, Republic of Korea!
Osstem leads the implant industry.Osstem Implant is the first implant company that built and pioneered dental implant when South Korea was newborn in medical equipment industry.
Under to business philosophy of “contribute to better human health by providing dentists with better treatment”, we have innovated and exploited the road that no other took and raised dazzling achievement.
With the highest quality and competiveness, we expanded as a company that ranks first in Asia, Pacific region and fourth globally. We are leading the national market with 28 corporation in China, Japan ,USA, Germany and 70 sales network globally. Moreover, during 2017-2018 our brand became most used implant brand by dentists, taking spot as number one brand in implant fixture sales.
However, Osstem’s challenge does not stop here.
With supporting dental treatment by total dental solution, we are setting goal as becoming number one implant company by 2026. Further we will lead the world’s dentist industry and become digital dentistry leader by digital dentistry full lineup. Osstem’s challenge will never stop, reaching further and further as No.1 dental company.We aim to become the world’s best dental implant company.

- Implant Business
- Dental Equipment and Supplies Business
- Dental IT Business
- Training Session
“OSSTEM IMPLANT SYSTEM”
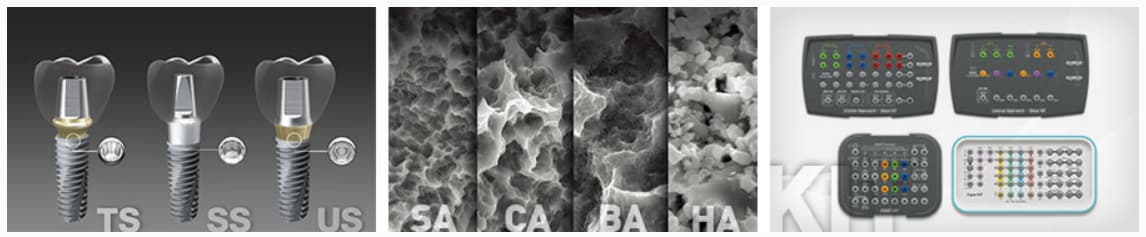
The main key to Osstem’s success to become a world-class implant company is our top quality implant products. We have been expanding R&D investment, and continuously carrying out quality innovation. As a result, we could gain a strong competitive edge in terms of technology and quality.
In particular, we have various kinds of implant systems that can be applied to various kinds of clinical cases. As a consequence of developing ultra-hydrophilic surface to improve osseointegration performance, as well as surface technology for weak bone quality, and launching various devices that make implant surgery safe and convenient, we have received much acclaim from our customers.
In addition, we have developed heterogeneous bone and synthetic bone for implant treatment in the case of insufficient bone mass, and also the GBR Tool to make the procedure easier.
Based on our outstanding product competitiveness, we are in the forefront of the development of new technologies and new products that will lead the global market.
- Main Markets
-
 Bolivia
Bolivia
 Colombia
Colombia
 Costa Rica
Costa Rica
 Dominican Rep.
Dominican Rep.
 Guatemala
Guatemala
- Main Product
- Attached File
Related Products

PRF BOX

Dental Implant Ratchet Hex Drivers

PRF BOX Implant

Sinus Lift Bone Expander Kit Dental

Implant system (SLA surface)



































![[크기변환]K3 design Dental Unit Chair K3](https://web.tradekorea.com/product/967/2075967_02/Dental_Unit_Chair_K3_2.png)