FREND COVID19 SP
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, poct, spike protein, covid test
- Category
- Medical Devices
NanoEnTek Inc.
- Recent Visit
- Jan 13, 2025
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
17



| Product name | FREND COVID19 SP | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Ingredients | - |
| Keyword | corona virus , poct , spike protein , covid test | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
Microfluidic Qualitative Immunoassay
FREND™ COVID-19 SP (Spike Protein)
The FREND™ COVID-19 SP is
- a point-of-care testing (POCT) which can be used to check whether patient has developed immune
response to SARS-CoV-2 using human serum or plasma.
- a fluorescence immunoassay (FIA) using the FREND™ System intended for the
qualitative detection of IgG and IgM antibodies to SARS-CoV-2.
- is intended for use as an aid in identifying individuals with an adaptive immune response
to SARS-CoV-2, indicating recent or prior infection.
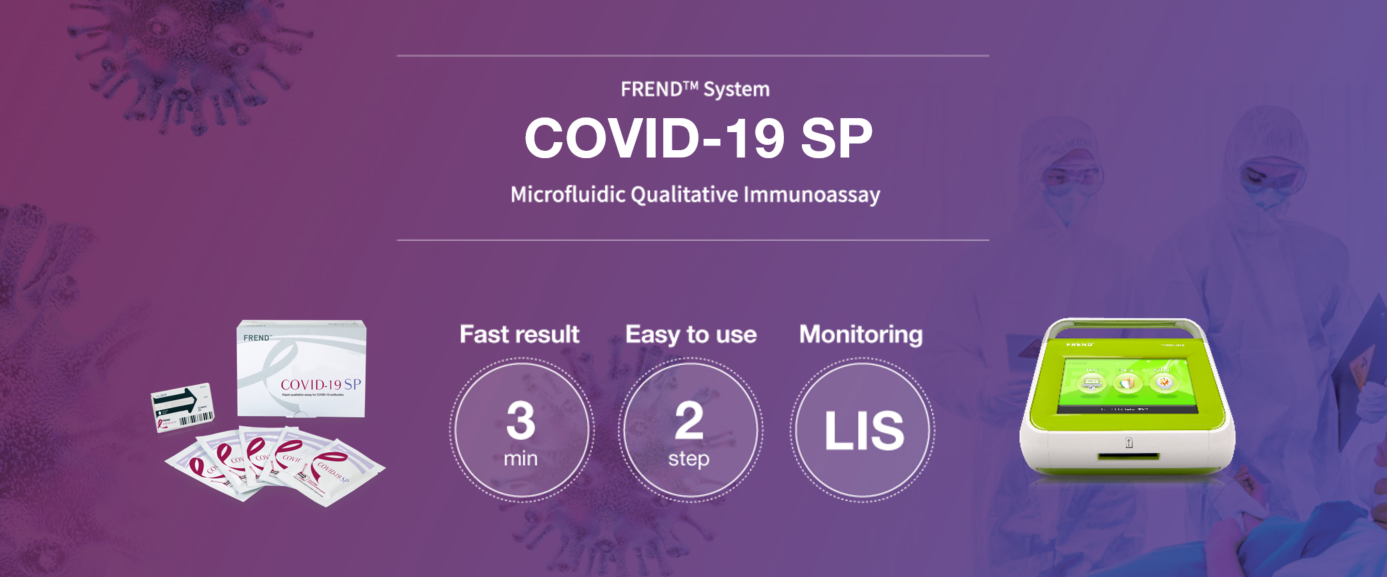
KEY FEATURES & BENEFITS
- 3 minutes | Fast result
- 2 steps | Easy to use
- 97% & 96% | Positive & Negative Percent Agreement
- Microfluidic Qualitative Immunoassay (spike protein)
- LIS connectivity (data management)
Instructions for use
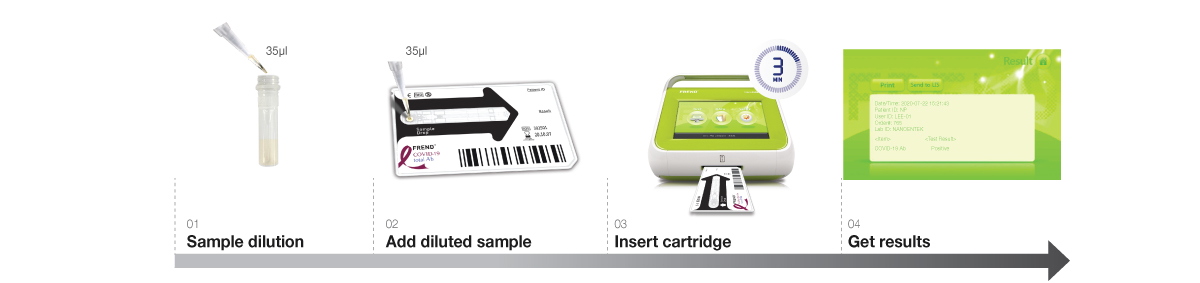
Accurate result in 3 minutes!
With just simple steps of operation, the FREND™ system supports quick decision-making.
Performance evaluation
Clinical agreement study
The total of 88 clinical samples (34 positive and 54 negative) confirmed with RT-PCR were tested
with the FREND™ COVID-19 SP. It shows 97.06% PPA (Positive Percent Agreement) and 96.30% NPA
(Negative Percent Agreement) as shown below.

Performance evaluation
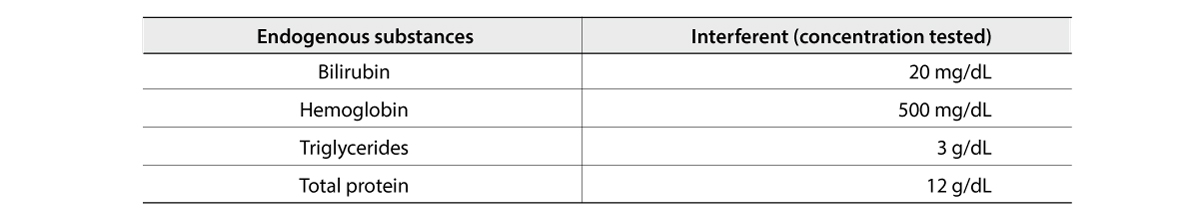
Interference
The interference evaluation test of FREND™ COVID-19 SP was conducted according to CLSI Guidelines EP7-A2 using one lot.
No interference in the testing of the FREND™ COVID-19 SP with 4 interfering substances was observed.
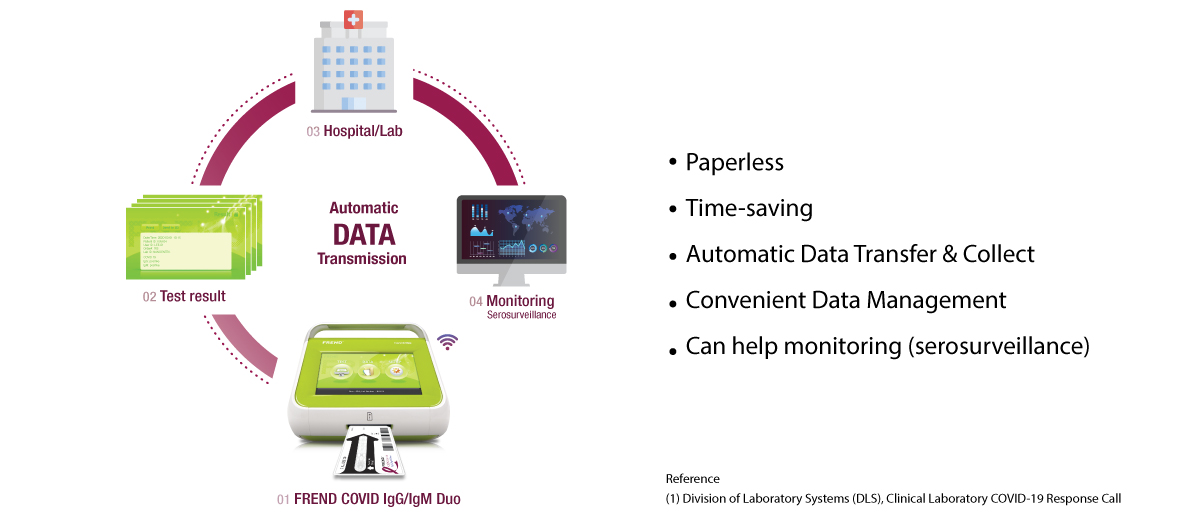
Effective Data Management
Laboratory Information System Connectivity
In the event of a pandemic, the management of a vast amount of clinical result is important.
However, many laboratories face the challenge in arranging the essential information effectively(1).
The FREND™ System which is LIS compatible provide following features:

Notification
• This test has not been reviewed by the FDA.
• Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
• Results from antibody testing should not be used as the sole basis to diagnose
or exclude SARS-CoV-2 infection or to inform infection status.
• Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains,
such as coronavirus HKU1, NL63, OC43, or 229E.
• Not for the screening of donated blood.
For more information, please visit www.nanoentek.com
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product
Related Products

Dental Implant Ratchet Hex Drivers

Stryker 1088 HD Camera Control

eyelash essence

Implant system (SLA surface)

Catch Me Patch Her's Band