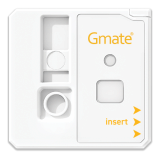
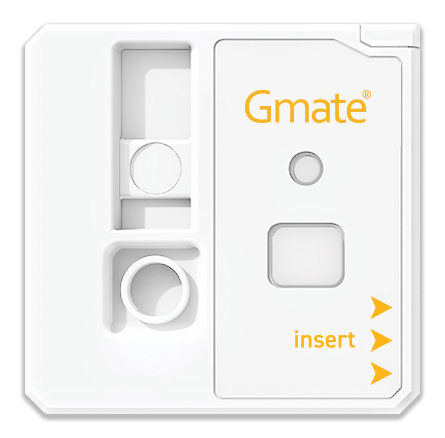
CORONA VIRUS ANTIGEN TEST ANALYZER GMATE COVID-19
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Philosys Healthcare Co., Ltd.
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, gmate, covid-19, antigen test
- Category
- Medical Analyzer
PHILOSYS
- Verified Certificate
-
16



| Product name | CORONA VIRUS ANTIGEN TEST ANALYZER GMATE COVID-19 | Certification | CE |
|---|---|---|---|
| Category | Medical Analyzer | Ingredients | - |
| Keyword | corona virus , gmate , covid-19 , antigen test | Unit Size | 34.1 * 5.6 * 33.75 mm |
| Brand name | Philosys Healthcare Co., Ltd. | Unit Weigh | 5 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 9027801000 |
Product Information
The Gmate® COVID-19 analyzer utilizes advanced surface treatment technology to maximize sensitivity for fast, accurate corona virus testing.
The Gmate® COVID-19 test cartridge is based on a magnetic force-assisted electrochemical sandwich immunoassay for the qualitative analysis. When sample such as nasal swab, nasopharyngeal swab specimens and expectorant is injected, automated sandwich immunoassay driven by dynamic magnetic fields.
Kindly refer to the video clip in the link below
For COVID-19 test, RT-PCR is widely used as the confirmation device for detecting COVID-19 but still has difficulties of testing with RT-PCR as below ;
Results may vary depending on ;
1) Sample collection and transportation conditions.
2) Laboratory conditions and researcher skill level.
3) Test adequacy (it takes more than 6 ~ 8 hours to get the result)
Also the cost of testing is relatively high. (need to have testing kits + reagent + infrastructure of laboratory + analyzing system)
Using Rapid kit (antibody IgG, IgM) could reduce time for testing but it has limits to be the best method, mainly because of the Low Accuracy.
1) Lowest accuracy: 30%
2) Only can detect the virus after antibody formation which comes after viral invasion.
3) Barely defects during incubation period which means unreliable for using it alone. (cannot detect virus in the first 5~7days of incubation period)
Please check the world wide problems with using cheap Chinese rapid test kits in the below news link.
These kind of rapid kits make the problem even worse.
Gmate COVID-19 rapid test kit (Ag)
We, Philosys received support from Korean Government and developed Gmate COVID-19 which rapidly detects Coronavirus directly from nasal swab less than 10 minutes.
Gmate COVID-19 is used for confirmation device for detecting COVID-19 with high accuracy (97.2%) which equivalent to those of final test as RT-PCR (95%) but less than “15 minutes.”
So, Gmate COVID-19 is the PCOT system (based on Antigen test) which you can get the accurate test result immediately at the testing site.
In detail, please refer to the comparison chart below. (also attached)
In fact, our system is being used in the area like ;
-Hospitals
-Airports and Seaports
-Sports stadium.
-labour site : construction site, call centers etc …
In terms of cost, Antibody rapid kit (IgG & IgM) is would be cheaper.
However, antibody test cannot detect the virus in the early stage of virus invasion. (antibody can only be detected 10~14days after the virus and symptom onset)
This is the major problem and very risky to manage the disease. There are many adverse cases in the news.
Antibody test can only be used to make decision in the final stage of infection that if patient is healthy enough and can be released from the isolation.
Detection method
Step 1. A protein in the specimen reacts with magnetic nanoparticles
Step 2.The magnetic field emitted by the device drives nanoparticles to form an immunized complex.
Step 3. Magnetic particles are used to remove non-reactive nanoparticles
Step 4. Measure the electroche3mical signal of the nanoparticles bound to the electrode
**Features
- Specificity: 100%
- Sensitivity: 94.4%
- Accuracy: 97.2%
- Simple and Fast Detection System : Results can be delivered within 15 min
**Certificate
: ISO
: CE
: Expected to receive the EUA-FDA in Sept.
Thank you.
**Features
- Simple and Fast Detection System : Results can be delivered within 15 min.
- The Purpose of our detection system is a Point of Care Testing.
- Gmate COVID-19 supports rapid testing of patients and distinguishes regular flu patients and Covid-19 patiens.
• Dimensions
- Dimension : 34.1 X 33.75 X 5.6 (mm)
- Weight: 5(g)
• Environmental Condition
- Storage Condition
: Temperature: 2~8C˚ (35.6 ~ 46.4˚F )
- Operating Condition
: Temperature: 18~28 C˚ (64.4~82.4 ˚F)
• Shelf Life
- Un-opened 12 months
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
- President
- CHOI IN HWAN
- Address
- 548-6, Gyeongjang-dong, Gunsan Si, JEOLLABUK-DO, 573-420, KOREA
- Product Category
- Glucose Meter
- No. of Total Employees
- 1-50
- Company introduction
-
PHILOSYS
- Main Product


































 South Korea
South Korea