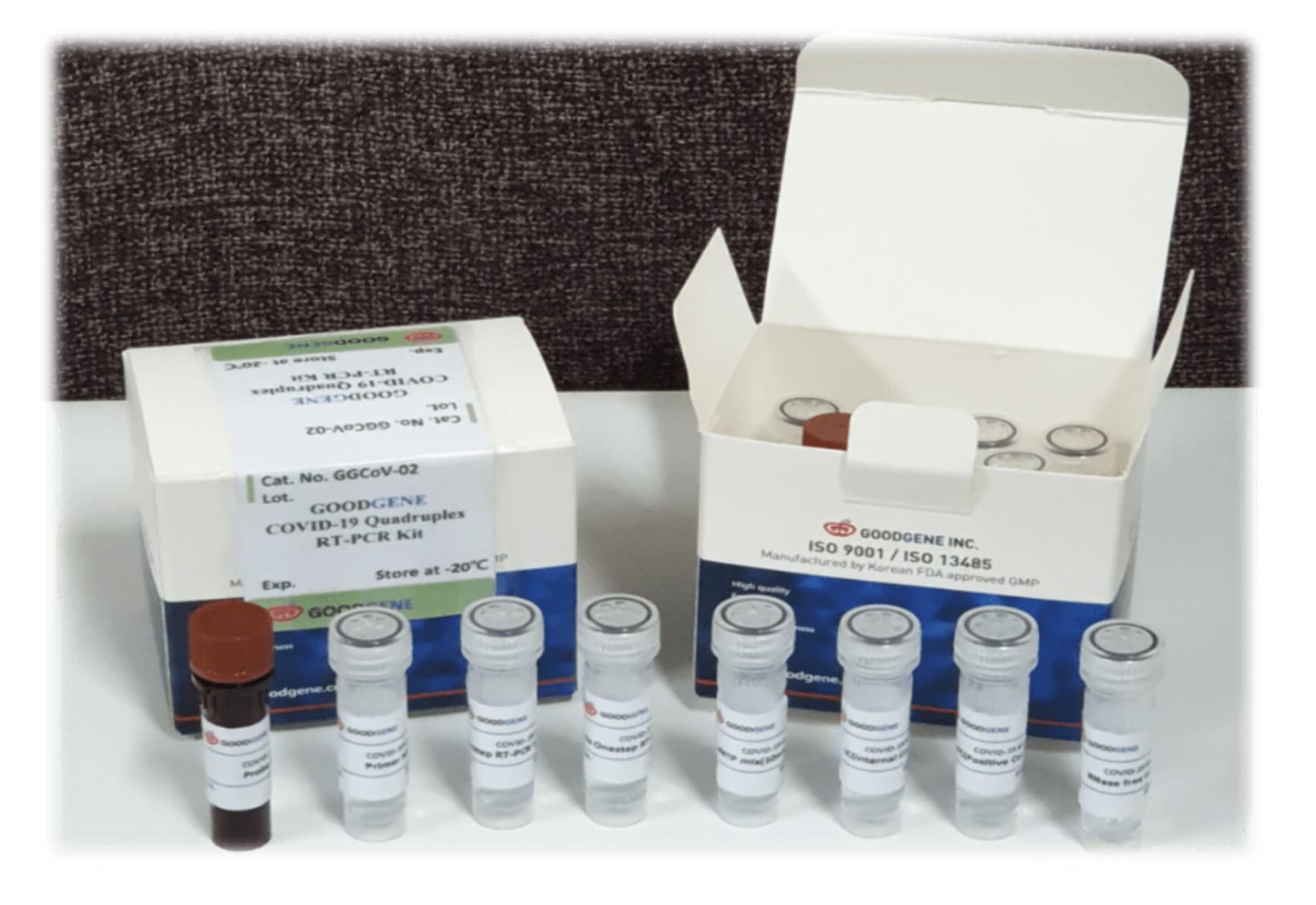
GG COVID-19 Quadplex Detection Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- D/A,D/P,L/C,MoneyGram,Others,T/T,Western Union
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- virus, detection kit, coronavirus, corona-19
- Category
- Medical Test Kit
GOODGENE INC
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
17


| Product name | GG COVID-19 Quadplex Detection Kit | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | virus , detection kit , coronavirus , corona-19 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 38220010 |
Product Information
The GoodGene GG COVID-19 quadplex reverse transcription Real time PCR Kit is based on Taqman probes and primers for 3 genes of SARS-CoV-2 and internal control (IC), beta-actin. Reverse transcription and real time PCR are carried out sequentially in the same tube. Thus it is a 4-Plex assay performed in single step and within a single tube.
It intends to maximize accuracy of detecting SARS-Cov-2 by testing not 1 or 2 but 3 genes of SARS-Cov-2 (minimizing risk of false negative due to mutation) and including IC in each reaction.
All components required for reaction are added during setup, and there is no need to add additional components once the reaction has been started, which is a very fast and will give satisfactory results in most cases. Its procedure is very simple and takes only 100 min.
This is a perfect standard test for high complexity laboratory specialized for COVID-19 test.
In vitro diagnostic (IVD) medical device for COVID-19 (Medical prescription (Rx) required)
Indicated in Persons Under Investigation (Individuals suspected of COVID-19 by their health care provider)
Provides qualitative detection of SARS-Cov-2 by Reverse-transcription Real Time PCR assay from human respiratory samples, including nasopharyngeal or oropharyngeal swabs or washing, sputum, bronchial alveolar lavage fluid and tracheal aspirates.
Intended Use :
In vitro diagnostic (IVD) medical device for COVID-19 (Medical prescription required) Indicated in Persons Under Investigation (Individuals suspected of COVID-19 by their health care provider) Provides qualitative detection of SARS-Cov-2 from human respiratory samples.
Storage:
Store at dark space at -15 to 200C.
Product validity period:
6 months from the date of production/ 1 month from first unpacking and thawing of the reagents.
Avoid excessive freeze/thaw cycles for reagents.
Specimen Type:
nasopharyngeal or oropharyngeal swabs or washing, sputum, bronchial alveolar lavage fluid and tracheal aspiratescollected according to standard technique and immediately placed in 1-3 mL of transport media.
Specimen handlings:
Specimens can be stored at 4 ℃ for up to 72 hours after collection. If any delay in extraction is expected, store specimens at -70℃ or lower. Extracted nucleic acids should be stored at -70 ℃ or lower
Amplification and Detection:
4 or more color real PCR instrument (ex. Bio-Rad, ABI 7500, RotorGene/Qiagen)
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | D/A,D/P,L/C,MoneyGram,Others,T/T,Western Union | Shipping time | Negotiable |
- President
- Moon Woo Chul
- Address
- 222-12, Guro-dong, Guro-gu, SEOUL, 152-050, KOREA
- Product Category
- Other
- Main Product
Related Products

R-ligo

Urine Analysis Test Strip Self-Stik Series
Covid 19 Detection

Self-Stik Urine Strips
BioTracer FOB Rapid Test