COVID-19 qPCR Multi Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- 1copy™ COVID-19 qPCR Multi Kit
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, covid-19 multi kit, pcr multi kit, corona tester
- Category
- Medical Test Kit

Moida
- Verified Certificate
-
5


| Product name | COVID-19 qPCR Multi Kit | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | corona virus , covid-19 multi kit , pcr multi kit , corona tester | Unit Size | 74.0 * 74.0 * 67.0 mm |
| Brand name | 1copy™ COVID-19 qPCR Multi Kit | Unit Weigh | 250 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 382200 |
Product Information
COVID-19 qPCR Multi Kit
1. Description
1copy™ COVID-19 qPCR Multi Kit provides the fast and accurate testing
solution for SARS-CoV-2, specifically targeting the E gene for beta
coronavirus and the RdRp gene for SARS-CoV-2 in nasopharyngeal swab
and oropharyngeal swab.
Our COVID-19 Real-Time PCR assay is based on the WHO & CDC reference
method and it has been carried out the in silico analysis for all registered
SARS-CoV-2 sequence database.
2. Intended Use
1copy™ COVID-19 qPCR Multi Kit is an in vitro real-time RT-PCR test for
qualitative detection of the E gene and RdRp gene of SARS-CoV-2 extracted
RNA from nasopharyngeal swab and oropharyngeal swab from individuals
with signs and symptoms of infection who are suspected of COVID-19.
The patients being tested meet the CDC SARS-CoV-2 clinical criteria.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA
is generally detectable in nasopharyngeal swab and oropharyngeal swab
during the acute phase of infection. Positive results are indicative of active
infection.
Negative results do not preclude SARS-CoV-2 infection and should not
be used as the sole basis for patient management decisions. Negative
results must be combined with clinical observations, patient history, and
epidemiological information.
The 1copy™ COVID-19 qPCR Multi Kit is intended for use by trained clinical
laboratory personnel specifically instructed and trained in the techniques
of real-time PCR and in vitro diagnostic procedures.
1. U.S. FDA emergency approved products
2. Total measurement time (1hour 50minutes), Hands-on time (20 minutes)
3. High accuracy reliability
4. Products in use in 12 countries around the world
5. 1copy™ COVID-19 qPCR Multi Kit provides the fast and accurate testing
solution for SARS-CoV-2, specifically targeting the E gene for beta
coronavirus and the RdRp gene for SARS-CoV-2 in nasopharyngeal swab
and oropharyngeal swab.
6. Comprehensive inspection system available
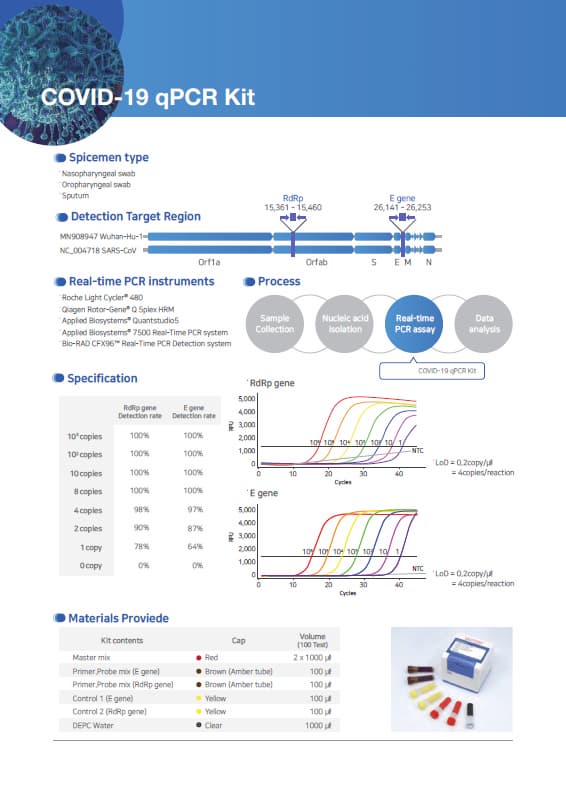
Materials Provided
Kit contents Cap color Volume(100 Test)
Master mix Red 2 x 1000 ㎕
Primer/Probe mix 1(E gene) Brown (Amber tube) 100 ㎕
Primer/Probe mix 2(RdRp gene) Brown (Amber tube) 100 ㎕
Control 1 (E gene) Yellow 100 ㎕
Control 2 (RdRp gene) Yellow 100 ㎕
DEPC DW Clear 1,000 ㎕
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- SEO JONG BOK
- Address
- 322 Byeollae 3-ro Namyangju-si Gyeonggi, Namyangju-si, Gyeonggi-do, Korea
- Product Category
- Face Mask,Other Facial Care,Other Health Care Products,Toothbrush Sanitizer
- Year Established
- 2016
- No. of Total Employees
- 1-50
- Company introduction
-
ㅇ 세계적으로 K방역의 관심 고조로 K방역용품의 문의 쇄도.
- 상반기 무역협회를 통해 문의만도 약 20여건으로 상반기 샘플전달을 통한 제품 신뢰도 증빙
- 하반기 수출주문 기대 하고 있습니다.





○ 동남아시아, 동남아 바이어 네트워크
바이어 초청 및 상담을 통해 수출 기반 확보

○ 중국 저장성 박람회 참석

○ 피부관리 솔루션
- 식물성원료와 단백질을 함유한 제형의 제품으로 친환경 원료를 사용한 프리미엄 토탈 관리 제품
- 수분과 영양공급을 동시에 할 수 있으며 유효성분을 피부 피부속까지 전달하고 수분 유지력을 높여줌


- Main Markets
-
 Indonesia
Indonesia
 Panama
Panama
 U.S.A
U.S.A
- Main Product
Related Products
Contour Test strips, Accu chek, One Touch, On Call Plus

I-STEM (Y-STEM) PRP KIT

Animal Urine Analysis Test Strip Self-Stik VET
BioTracer Troponin I Rapid Test
COVID 19 IgM / IgG RAPID KIT



































 South Korea
South Korea