COVID 19 IgM / IgG RAPID KIT
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- COVID – 19 IgM / IgG RAPID KIT
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, diagnosis, virus, covid 19

MaruAra
- Verified Certificate
-
6


| Product name | COVID 19 IgM / IgG RAPID KIT | Certification | - |
|---|---|---|---|
| Category |
Emergency & Clinics Apparatus
Medical Analyzer Medical Test Kit Other Examination & Testing Instrumnet |
Ingredients | - |
| Keyword | corona virus , diagnosis , virus , covid 19 | Unit Size | - |
| Brand name | COVID – 19 IgM / IgG RAPID KIT | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 382200 |
Product Information
ADTech COVID-19 IgM / lgG KIT is a gold nanoparticle based immunochromatography test kit that qualitatively measures IgM and IgG antibodies COVID-19 in whole blood, serum or plasma. The kit is accurate, easy to use, and results can be checked the naked eye within 10 ~ 15 minutes.
PRODUCT DESCRIPTION
ADTech
COVID – 19 IgM / IgG RAPID KIT
Model No.: C 0430-1
Quantity: 20 Tests / KIT
Sample: Venous / Finger blood, Plasma and serum.
Test time: Reading in 10 ~ 15 minutes
Storage: 2℃ ~ 30℃(35.6℉ ~ 86℉)
Expiry: 2 year since manufactured

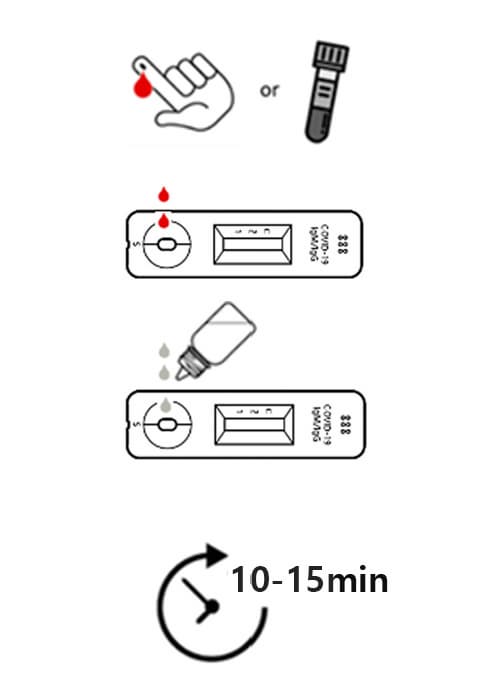
HOW TO USE
1. Collecting sample
For the test, 10㎕ of whole blood, plasma or serum is used. Collect the blood sample obtained by venipuncture into blood collection tubes or use fingertip blood.
2. Adding of sample
Add the collected sample into the sample inlet of the test cassette.
3. Dropping of sample buffer
Add 3 drops(90㎕) of sample into the inlet of the test cassette.
4. Reading the test results
Read the test result at 10 ~ 15 minutes.
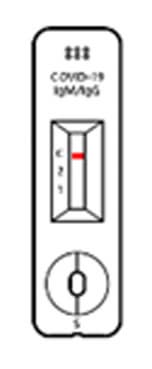
TEST RESULT
Positive
When all the 3 lines are shown at Test 1, Test 2 and control, then the test results is “POSITIVE”. Even in case 2 lines are shown at ‘control and Test 1’ or ‘Control and Test 2’, the test result is “POSITIVE”, too.
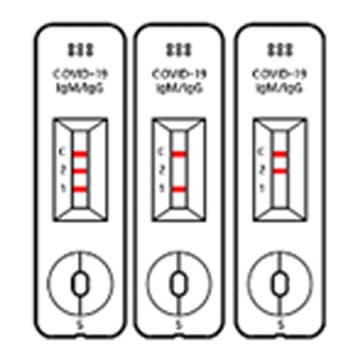
Negative
When the control line does show up without 2 Test lines, the test result is “NEGATIVE”.
※ If the line us not shown up at the control line though test if fulfilled, then that test cassette and test result are invalid, at that time, you have to re-test with new test cassette.
[Intended Use]
Final Check COVID-19 IgM / IgG test kit is an in vitro diagnostic medical device that qualitative detection IgM and IgG for 2019 novel coronavirus (2019-nCoV) in whole blood, serum or plasma by immunochromatography.
[Summary]
COVID-19 is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The origin of this virus is unknown, but it has been transmitted from person to person. It spreads mainly through drops that cough and sneeze. Common symptoms include fever, cough, and difficulty breathing. In most cases, minor symptoms develop, but some also progress to pneumonia and multi-organ failure.
Final Check COVID-19 IgM / IgG test kit can detect IgM and / or IgG antibodies from COVID-19 in the field.
The mouse anti-human IgM is immobilized on the IgM test line (“T1”) of the nitrocellulose membrane inside this test device, and the mouse anti-human IgG is immobilized on the IgG test line (“T2”). Serum, plasma or whole blood is added to the sample inlet of the test device, and then the sample dilution solution is sequentially dropped into the sample inlet of the test cassette. The
novel coronavirus IgM and / or IgG in the specimen reacts with the gold particle conjugated recombinant novel coronavirus protein and reacts with coated mouse anti-human IgM and / or
mouse anti-human IgG of nitrocellulose membrane. If the specimen contains the novel coronavirus IgM, the color appears on the test line “T1”. If the sample contains the novel coronavirus IgG, the color appears on the test line “T2”. If there is no novel coronavirus antibody in the specimen, the test line does not develop and only the control line develops. If no color appears on the control line, the inspection is considered invalid.
ADTech COVID-19 IgM/IgG
REF C0430-1 (20Test/Kit)
[Component]
1. Test device (sealed in aluminium pouch with desiccant)
2. Sample buffer
3. Alcohol swab
4. Capillary tube
5. Lancet
[Additional Required Equipment]
1. Micropipet
2. Timer
3. Disposable gloves
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Lee Jong Won
- Address
- 401ho 311 dash 8 Tanjungro, Ilsandong-gu, Goyang-si, Gyeonggi-do, Korea
- Product Category
- Other Gifts & Crafts,Skin Care Serum,Skin Toner
- Year Established
- 2019
- No. of Total Employees
- 1-50
- Company introduction
-
Welcome to MaruAra.
Our MaruAra is a company that collects and exports the best products that do not harm people and nature in Korea.
There are various conditions when selecting products, but the most important thing is to select products that do not harm people and nature.
No matter how good the product is, if it is harmful to the human and nature, we will not handle it.
MaruAra is pure Korean word, meaning sky and sea.
Our MaruAra only deals with products that are helpful to us and not harmful to nature, and we promise to treat only the best products in Korea.
- Main Product
Related Products

R-ligo
Contour Test strips, Accu chek, One Touch, On Call Plus

Nurugo CPR manikin
VTM, UTM, Viral Transport Media, EnTM Collection and Transport System
AFP/PSA/CEA Rapid Test


































 South Korea
South Korea