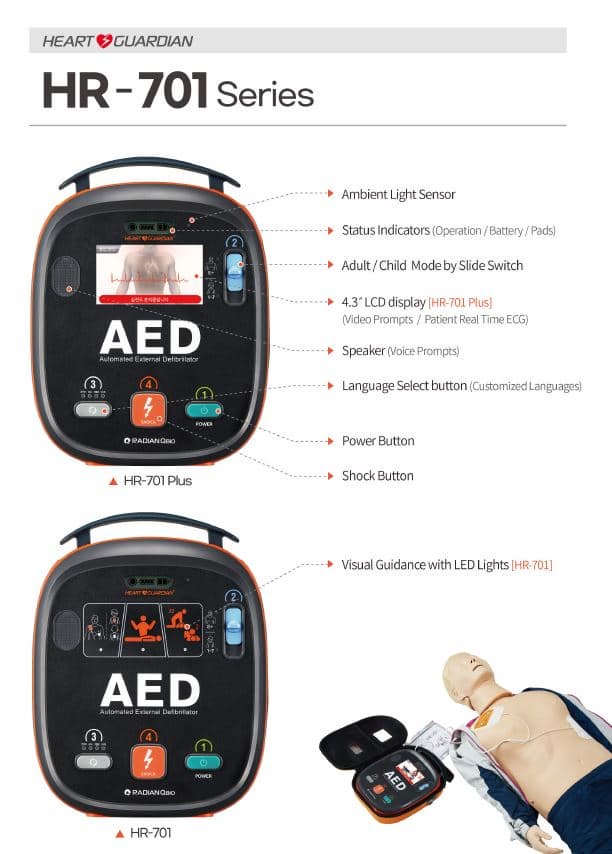
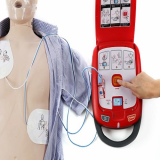
AED(Automated external defibrillator) Portable Defibrillator
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Heart Guardian HR-701 Series
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- aed, electrode pads, medical device aed automated external defibrillator, heart attact, sudden cardiac arrest, heart disease, first aid device, color lcd screen voice control bluetooth trainer cabinet aed cabinet customized aed simulation, cpr traning, korea defibrillator
- Category
- Medical Devices , Emergency & Clinics Apparatus

RADIANQBIO
- Verified Certificate
-
16



| Product name | AED(Automated external defibrillator) Portable Defibrillator | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Emergency & Clinics Apparatus |
Ingredients | - |
| Keyword | aed , electrode pads , medical device aed automated external defibrillator , heart attact , sudden cardiac arrest , heart disease , first aid device , color lcd screen voice control bluetooth trainer cabinet aed cabinet customized aed simulation , cpr traning , korea defibrillator | Unit Size | 280.0 * 222.0 * 74.4 mm |
| Brand name | Heart Guardian HR-701 Series | Unit Weigh | 2 kg |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 90189090 |
Product Information
RADIANQBIO Co.,
Ltd., a social corporation dedicated to saving lives, was established in
October 2005 and has been specialized in R&D, manufacture and sale of
sensor, medical devices, measuring equipment, tester, etc., over the last
decade.
Particularly, RADIANQBIO CO., Ltd., has opened up the market for medical and
healthcare business in 2014 based on its know-how and expertise amassed in the
field of precision measurement and analysis for many years of work, along with
its patented technology and technological capability of professional researchers.
RADIANQBIO Co., Ltd., successfully obtained domestic certification for its
automatic defibrillator which culminated about 2 years of R&D in tandem
with its localization and is currently proceeding with overseas certification
(CE and etc.) to make inroads into the global market.
We will lay the cornerstone for fusion and convergence industrial field as a
leader of creative economy while pushing forward ethical management that takes
safety and quality as top priority based on the slogan "Life of human is
the best value to uphold'
We will make utmost effort to ensure customer satisfaction by fully leveraging
our leading technology that sets apart from others.
*The objective of Participation
We, RadianQbio want to become the Medical health care specialist based on sensor technology
and support customer of health care will be diversified (Public -> Private)
and expect to see continued growth through EUKPP2016.
AED is recently obliged to install by support of government and to expand
oversea and domestic market. Our excellent quality, product design and
competitive price is worldwide satisfied. So far Medical device industry has
been driven by advanced countries (USA, Japan, Europe, etc.) based on the
latest technology, but currently competitive situation is happened due to their
increased production costs. We have high-technology comparing to the price
expectations over the overseas market.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- BEOM KI KIM
- Address
- 1804, Halla-sigma valley,453 gasan digital 2-ro,Geumcheon-gu,seoul,korea
- Product Category
- Medical Analyzer,Medical Devices,Medical Services,Medical Test Kit
- Year Established
- 2005
- No. of Total Employees
- 51-100
- Company introduction
-
[Company Introduction]
RADIANQbio Co., Ltd., a social corporation dedicated to saving lives, was established in October 2005 and has been specialized in R&D, manufacture and sale of sensor, medical devices, measuring equipment, tester, etc., over the last decade.
Particularly, RADIANQbio Co., Ltd., ., has opened up the market for medical and healthcare business in 2014 based on its know-how and expertise amassed in the field of precision measurement and analysis for many years of work, along with its patented technology and technological capability of professional researchers. RADIANQbio Co., Ltd., successfully obtained domestic certification for its automatic defibrillator which culminated about 2 years of R&D in tandem with its localization and is currently proceeding with overseas certification (CE and etc.) to make inroads into the global market.
We will lay the cornerstone for fusion and convergence industrial field as a leader of creative economy while pushing forward ethical management that takes safety and quality as top priority based on the slogan "Life of human is the best value to uphold'
We will make utmost effort to ensure customer satisfaction by fully leveraging our leading technology that sets apart from others.
- Main Markets
-
 Greece
Greece
 Ireland
Ireland
 Italy
Italy
 Lithuania
Lithuania
 Luxembourg
Luxembourg
 Netherland
Netherland
 Poland
Poland
 Portugal
Portugal
 Spain
Spain
 Turkey
Turkey
- Factory Information
-
RADIANQBIO
- Main Product
Related Products

Modular type ENT workstation_XU5 Visual

Stryker 1088 HD Camera Control

Chitosan spunlace Nonwoven fabric for Medical Cosmetic

IMPLANT DENTAL DRILLS IMPLANTS INSTRUMENTS

Hairview


































_Portable_Defibrillator_2.jpg)
_Portable_Defibrillator_3.jpg)
_Portable_Defibrillator_4.jpg)
 South Korea
South Korea
_Portable_Defibrillator_2.jpg)

