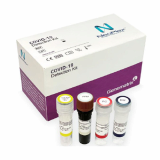
Covid 19 Detection
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- NeoPlex 2019-Novel Coronavirus COVID-19 Detection
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, detection, virus, covid 19

MaruAra
- Verified Certificate
-
5


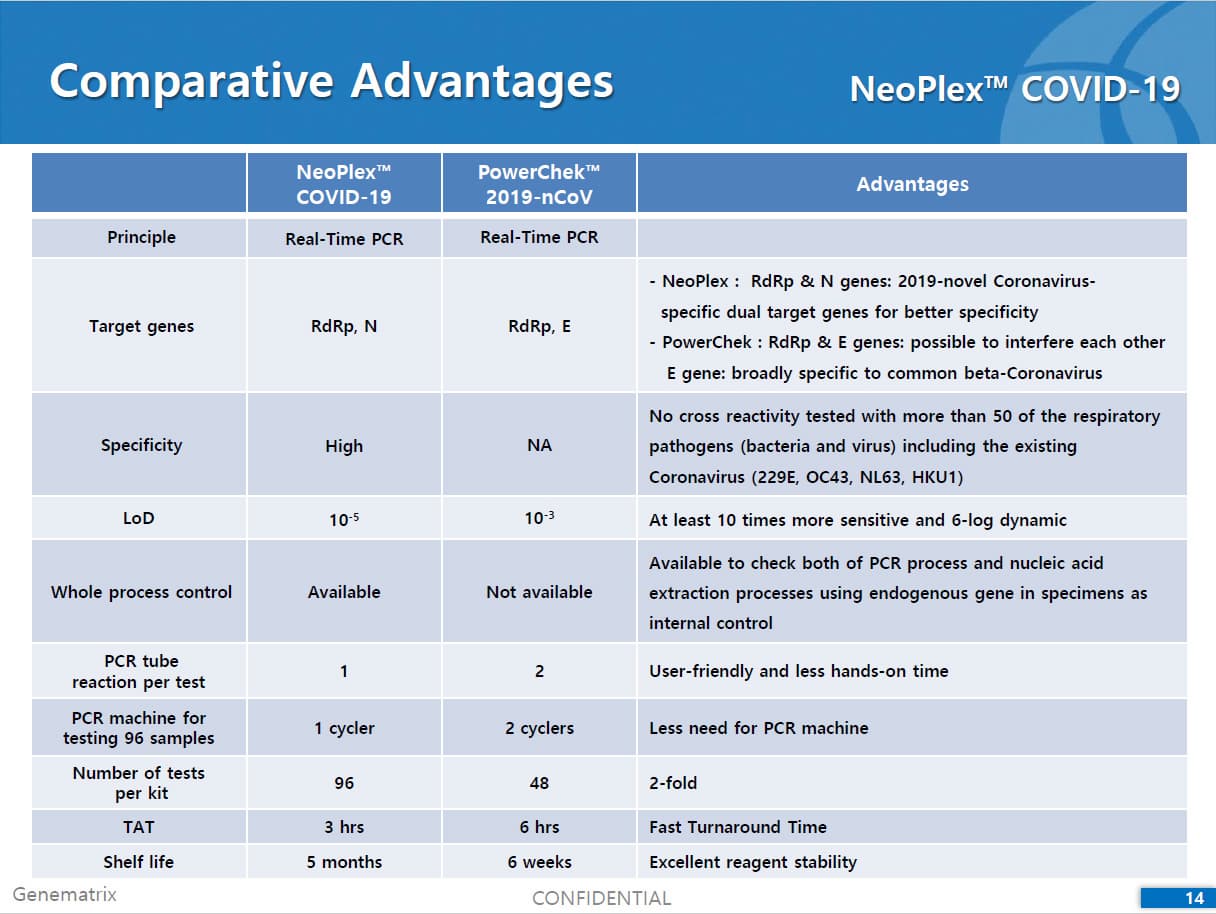
| Product name | Covid 19 Detection | Certification | CE , FDA |
|---|---|---|---|
| Category |
Emergency & Clinics Apparatus
Medical Analyzer Medical Test Kit Other Examination & Testing Instrumnet |
Ingredients | - |
| Keyword | corona virus , detection , virus , covid 19 | Unit Size | - |
| Brand name | NeoPlex 2019-Novel Coronavirus COVID-19 Detection | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 3822001020 |
Product Information
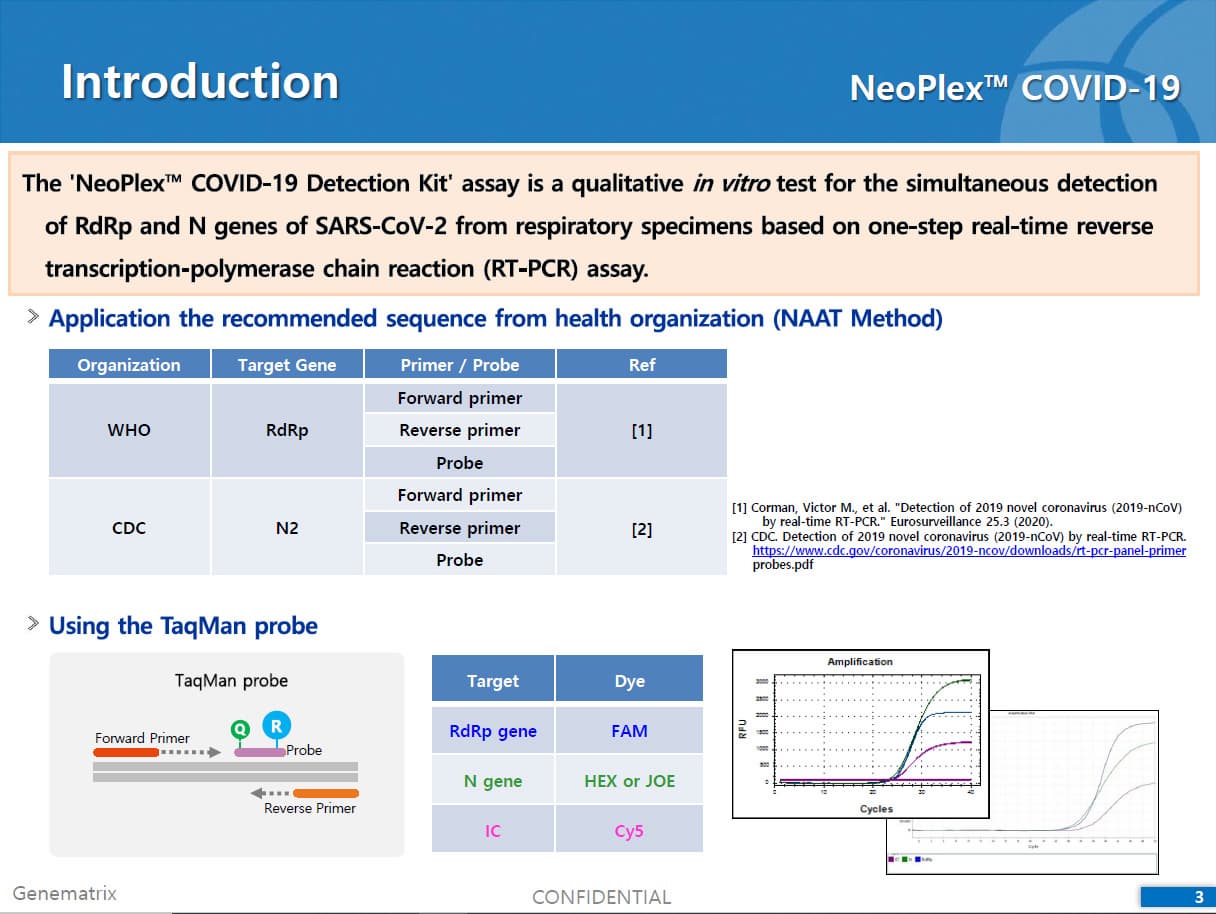
The 'NeoPlex COVID-19 Detection Kit' assay is a qualitative in vitro test for the simultaneous detection of RdRp and N genes of SARS-CoV-2 from respiratory specimens based on one-step real-time reverse transcription-polymerase chain (RT-PCR) assay.
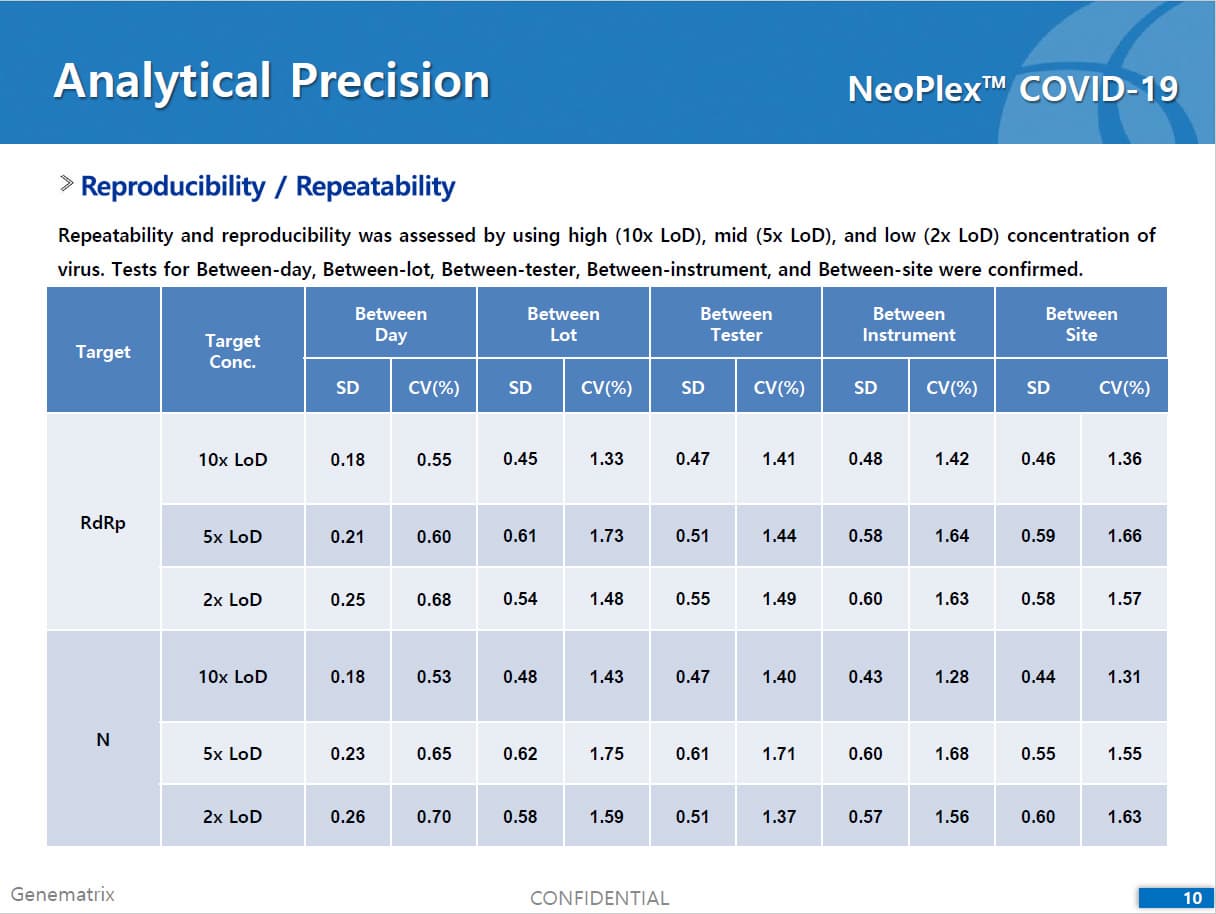
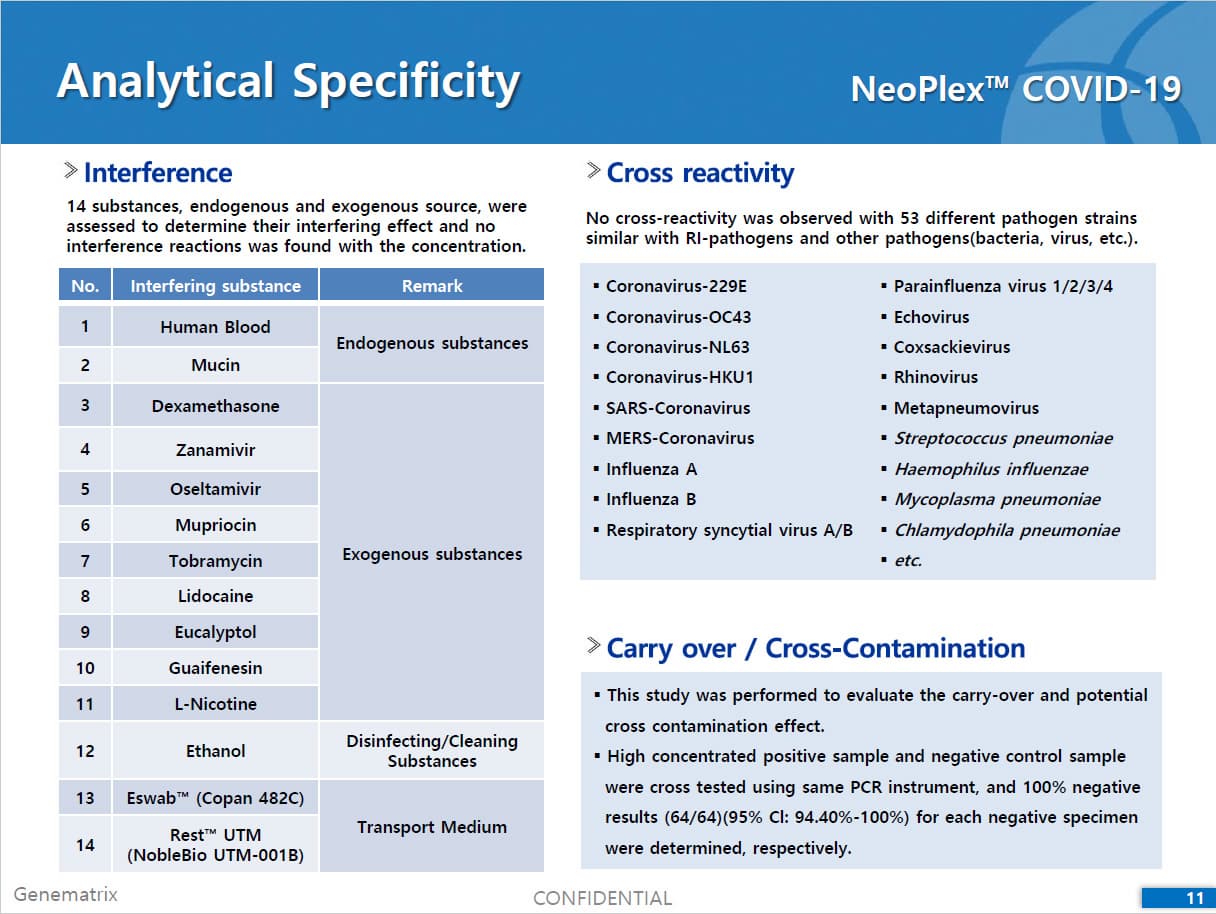
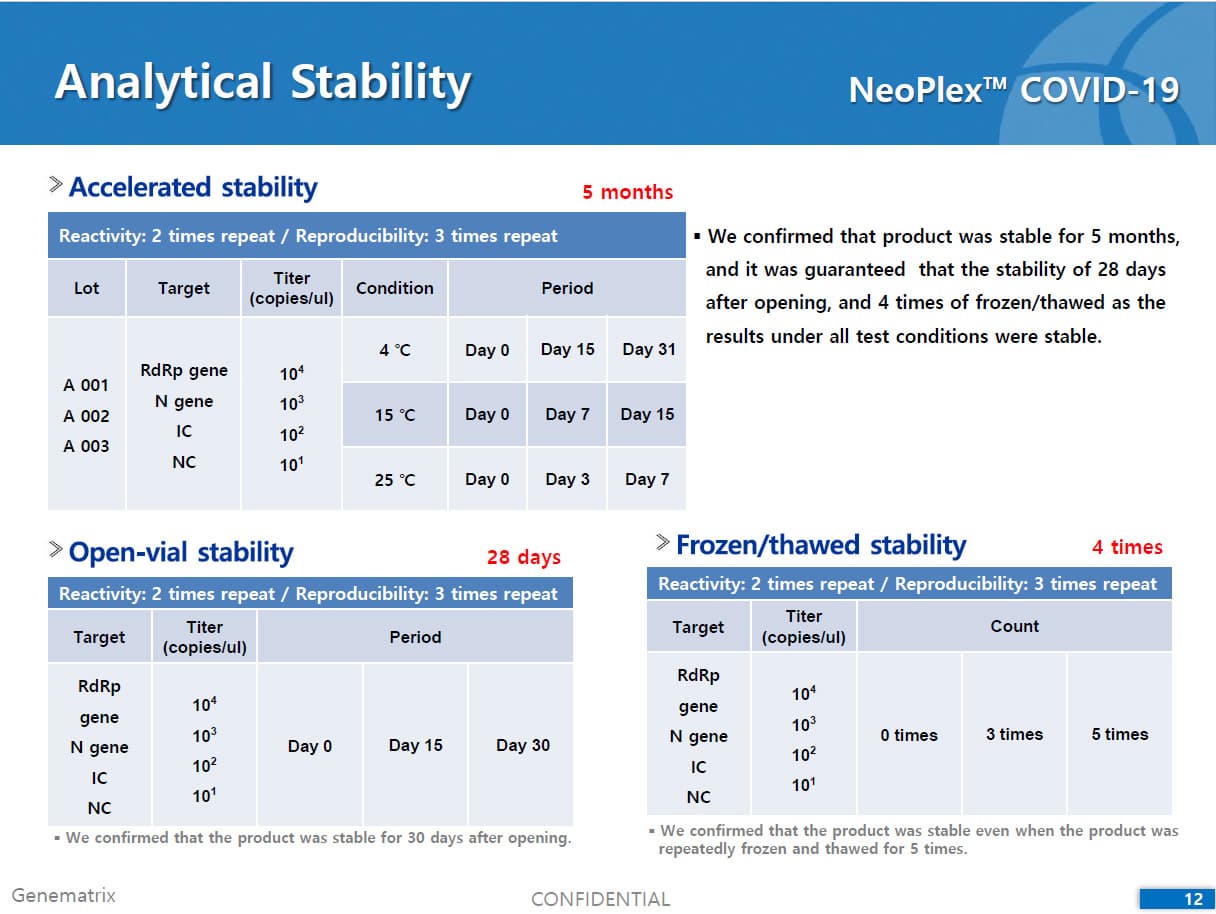
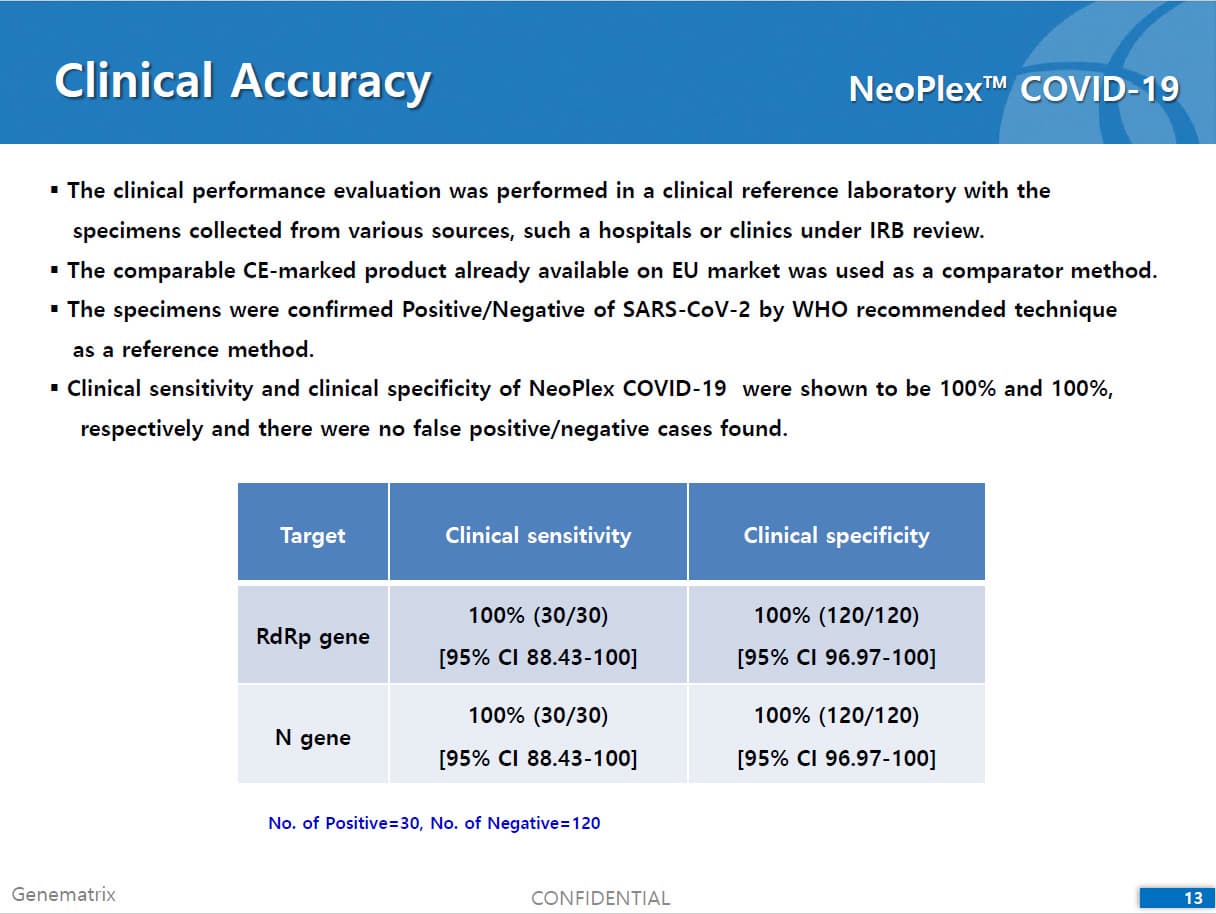

• C Tag TM technology * is the proprietary platform technology of GeneMatrix that enables simultaneous analysis of multiple pathogens in a single tube reaction. It is a next generation real time PCR molecular diagnostic technology which overcomes the limitation of existing technology such as TaqMan by increasing the number of analysis per fluorescence channel or existing tagging methods such as Invader by improving the possible generation of non specific signals.
• C Tag TM Molecular Diagnostic Platform provides rapid and accurate results for disease causing multiple pathogens, thereby reducing costs, saving time and increasing convenience. Therefore based on prompt and accurate test results of infected pathogens, it is possible to achieve timely treatment
at the early stage so that optimal treatment outcomes can be expected.
GENERAL INFORMATION
INTENDED USE
The NeoPlex™ COVID-19 Detection Kit Assay is a real-time RT-PCR in vitro diagnostic test intended for the qualitative detection of RNA from SARS-CoV-2 isolated and purified from respiratory specimens* obtained from individuals who meet COVID-19 clinical and/or epidemiological criteria. The NeoPlex™ COVID-19 Detection Kit is for use only under Emergency Use Authorization (EUA) in the US laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C.§263a, to perform high complexity tests.
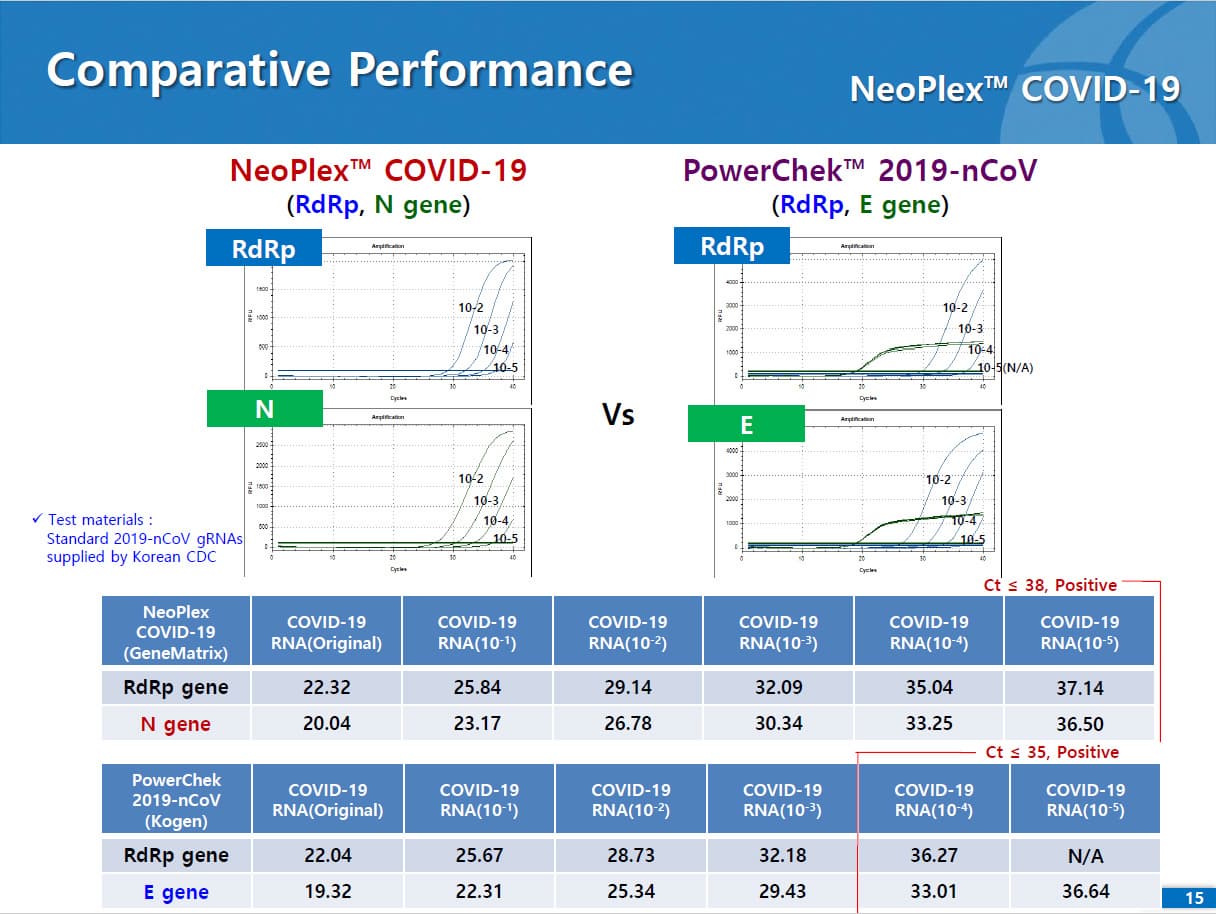
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in respiratory specimens* during the acute phase of infection. NeoPlexTM COVID-19 Detection Kit targets RdRp and N genes of SARS-CoV-2 as WHO and CDC recommended. As E gene is specific to both SARS-CoV-2 and common β-coronavirus (such as HKU1 and OC43) and was found to induce mutual interference with RdRp gene during the assay development, E gene is replaced with N gene in the assay. No cross-interference was detected between dual targets (RdRp and N genes), leading to improved sensitivity and specificity of the assay. Positive results are indicative of the presence of SARS-CoV-2 RNA, clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with other clinical observations, patient history, and epidemiological information.
The NeoPlex™ COVID-19 Detection Kit Assay utilizes Open Access reagents and functionality and is intended for use by trained clinical laboratory personnel specifically instructed and trained in the operation and in vitro diagnostic procedures. The NeoPlex™ COVID-19 Detection Kit Assay is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Summary and Explanation of the Test
SARS-CoV-2 is a novel coronavirus belonging to the family of Coronaviruses and causes coronavirus disease 2019(also called COVID-19). Beginning in December 2019 in Wuhan City, China, SARS-CoV-2 has been spreading globally and World Health Organization (WHO) declared the spate of infections caused by SARS-CoV-2 a pandemic on March, 2020. More than 350,000 people were confirmed as SARS-CoV-2 infected and 15,000 people were dead currently. The most common symptoms are fever, cough, fatigue, and shortness of breath. However, individuals can develop severe symptoms including pneumonia or respiratory failure.
The NeoPlex™ COVID-19 Detection Kit Assay is a qualitative in vitro test for the simultaneous detection and confirmation of N gene and RdRp gene in 2019-Novel Coronavirus causing COVID-19 from respiratory specimens* based on real-time reverse transcription polymerase chain reaction(RT-PCR) assay. This test kit is intended for professional use.
Principles of the Procedure
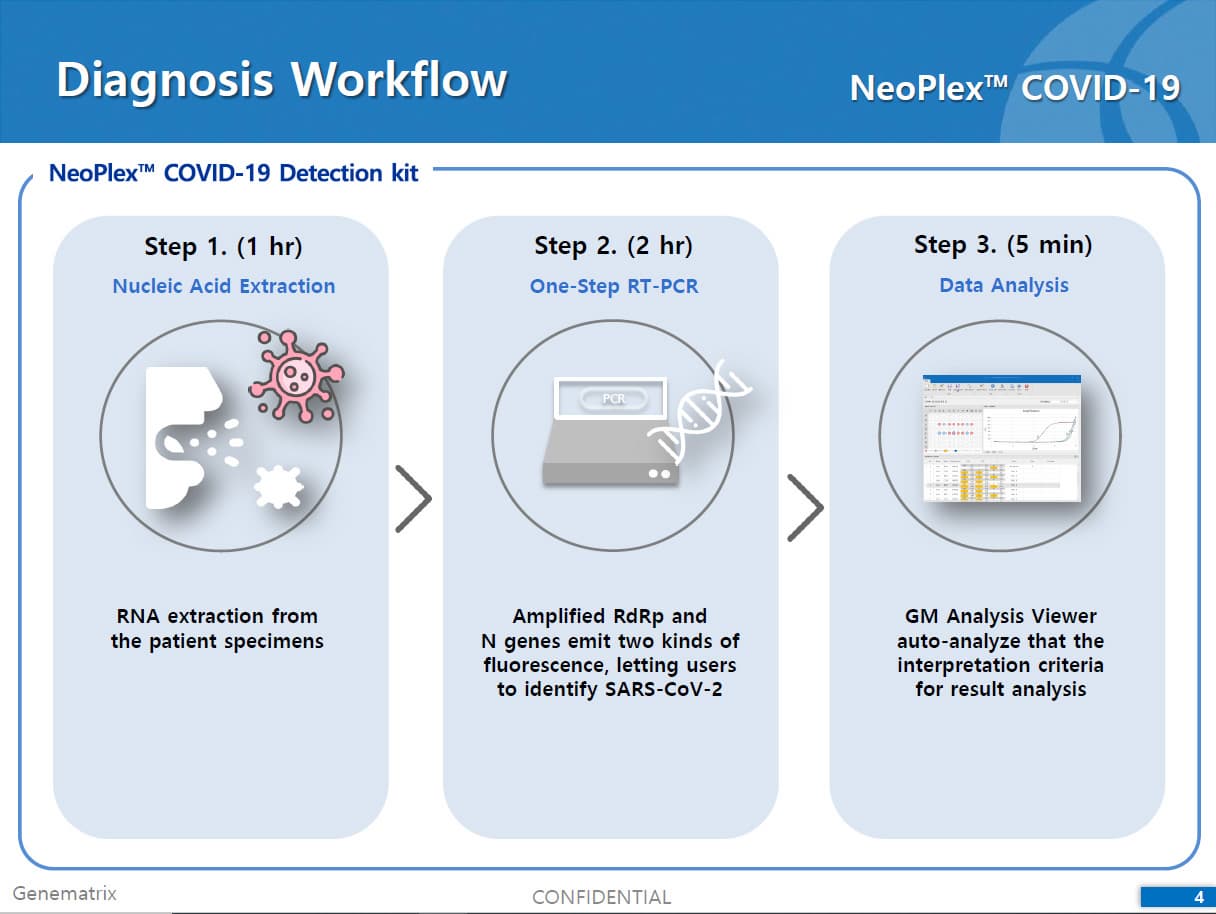
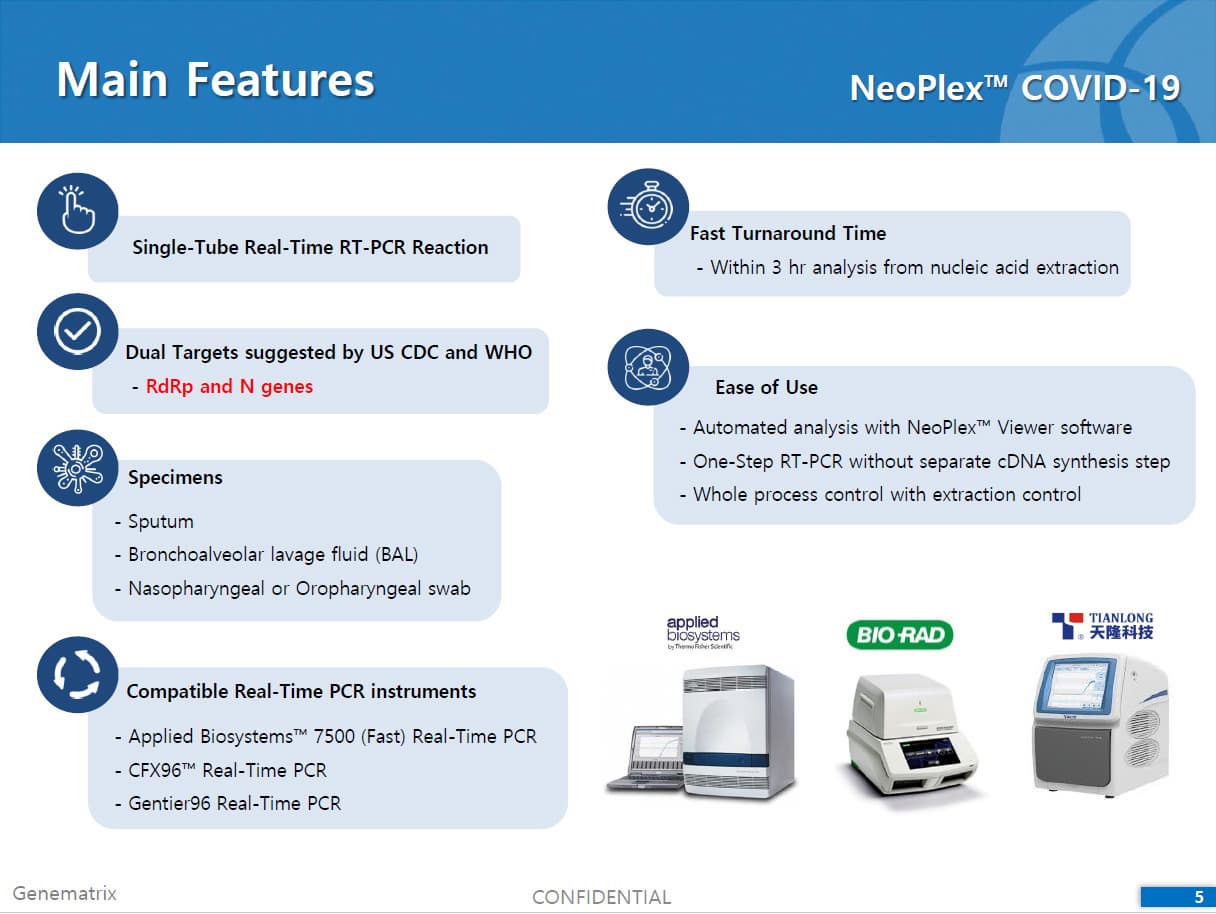
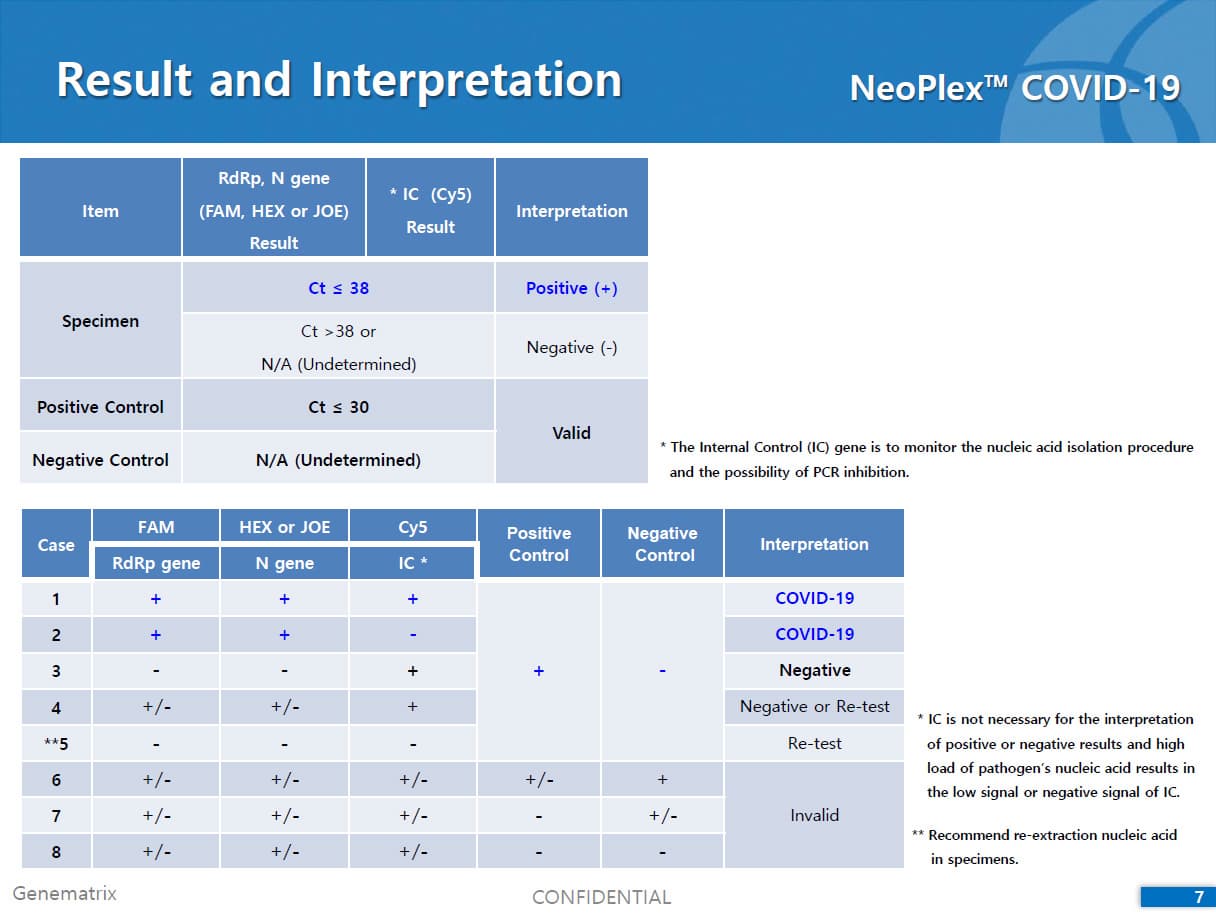
The test is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in respiratory specimens (eg. Sputum, Nasopharyngeal or Oropharyngeal swab). NeoPlexTMCOVID-19 Detection Kit is based on two major processes; 1) Isolation of nucleic acid from specimens, 2) Multiplex real-time amplification. The primer & probe system is based on the standard TaqMan® Technology. The SARS-CoV-2 specific probes are labelled with the FAM fluorophore and HEX fluorophore to target COVID-19 RdRp gene and N gene, respectively. The internal control is labelled with the Cy5 fluorophore.
1) Isolation of nucleic acid from specimens: Nucleic acid can be extracted from specimens by automated purification system or using manual prep kits (QIAamp DSP Viral RNA Mini Kit or equivalent).
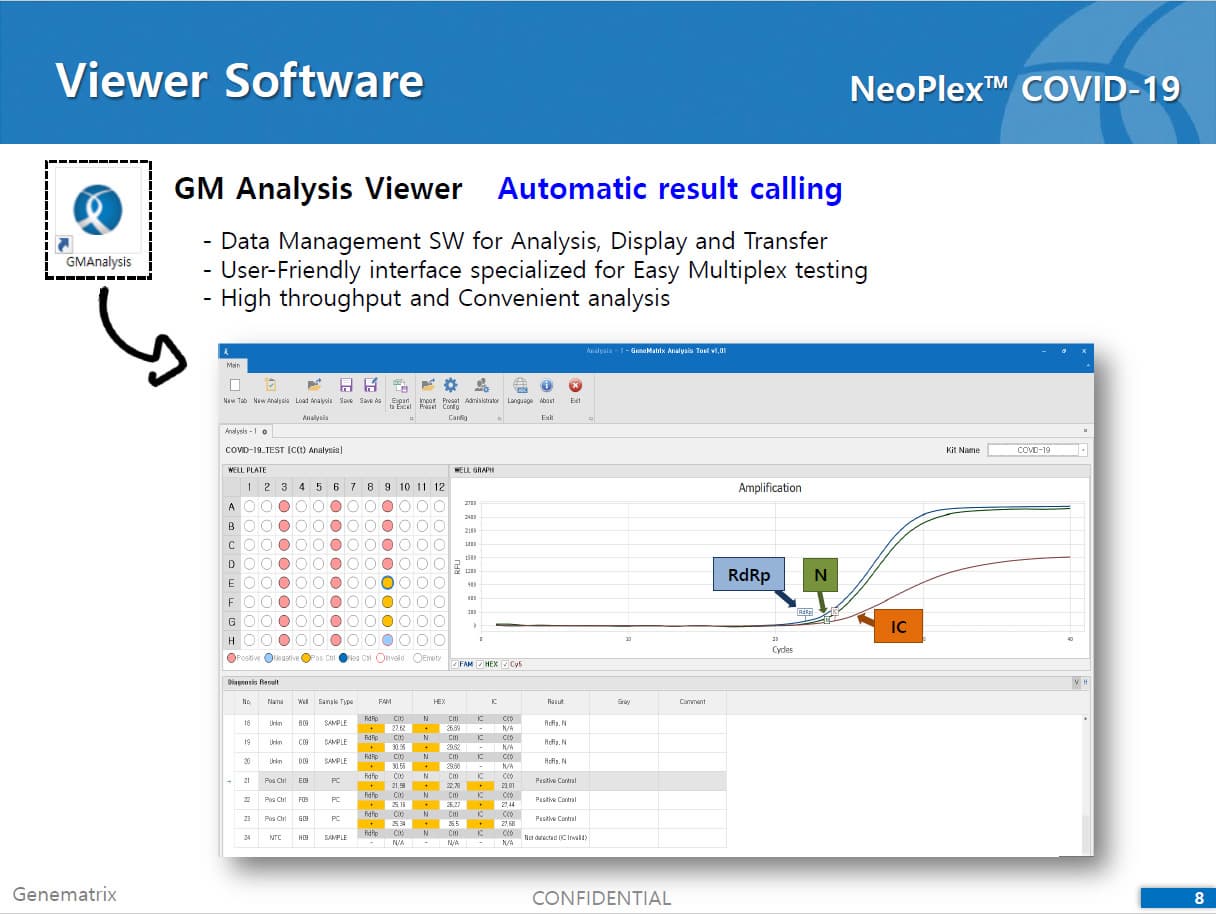
2) Mutiplex real-time PCR: RNA isolated from specimens is reverse transcribed to cDNA and subsequently amplified using one of the two Real-time PCR instruments (the Applied Biosystems™ 7500 (Fast) Real time PCR Instrument system (Thermo Fisher Scientific) or CFX96™ Real time PCR detection system (Bio-Rad). The assay includes an internal control to detect PCR inhibition and the integrity of the PCR run.
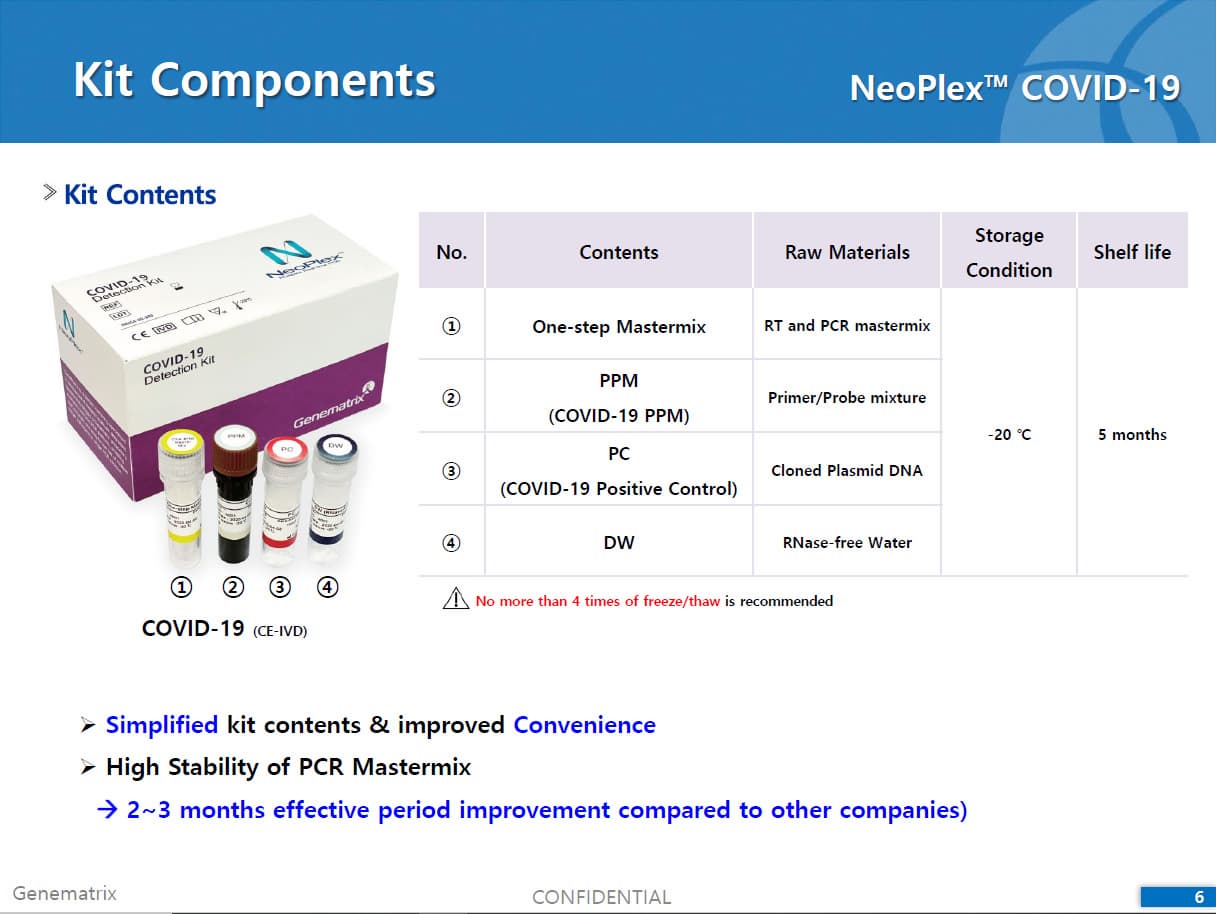
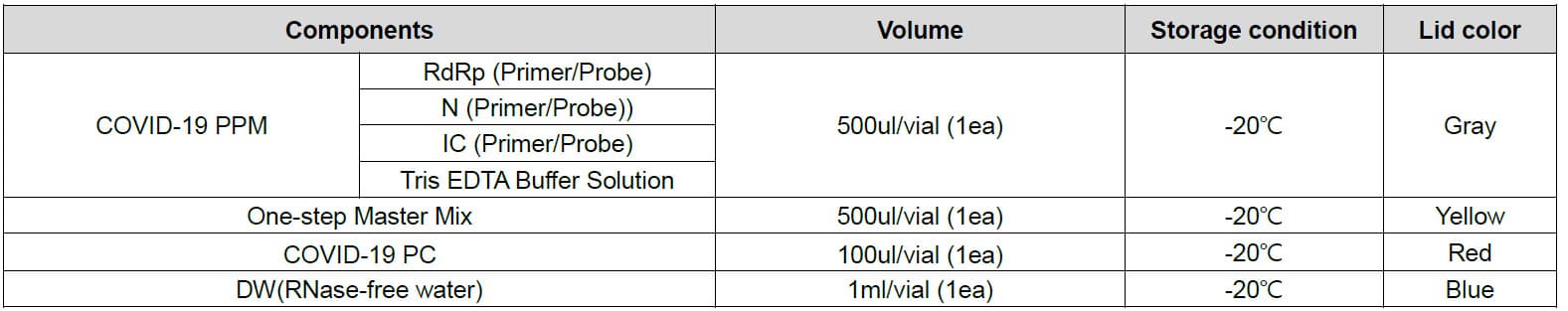
Materials Provided
The NeoPlexTMCOVID-19 Detection Kit contains sufficient reagents for 96 reactions and the components are as follow:
Materials Required but Not Provided
0.2 ml 8-Tube PCR Strips without Caps, low profile, white (Bio-Rad, Inc., Cat No. TLS0851)
Optical Flat 8-Cap Strips for PCR Tubes (Bio-Rad, Inc., Cat No. TCS0803)
QIAamp DSP Viral RNA Mini Kit (QIAGEN,Cat No.61904) or equivalent nucleic acid extraction kit
Pipettes set, P2/P10, P20, P200, and P1000 aerosol barrier tips
Compatible Real-Time PCR instruments
- Verified Certificate
-


B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Lee Jong Won
- Address
- 401ho 311 dash 8 Tanjungro, Ilsandong-gu, Goyang-si, Gyeonggi-do, Korea
- Product Category
- Other Gifts & Crafts,Skin Care Serum,Skin Toner
- Year Established
- 2019
- No. of Total Employees
- 1-50
- Company introduction
-
Welcome to MaruAra.
Our MaruAra is a company that collects and exports the best products that do not harm people and nature in Korea.
There are various conditions when selecting products, but the most important thing is to select products that do not harm people and nature.
No matter how good the product is, if it is harmful to the human and nature, we will not handle it.
MaruAra is pure Korean word, meaning sky and sea.
Our MaruAra only deals with products that are helpful to us and not harmful to nature, and we promise to treat only the best products in Korea.
- Main Product


































 South Korea
South Korea