Entecavir EP Impurity F CAS NO.:649761-24-0
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Pharmaceutical Intermediates
Hubei YoungXin Pharmaceutical Tech Co.,Ltd.
- Verified Certificate
-
6


| Product name | Entecavir EP Impurity F CAS NO.:649761-24-0 | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | cas no.:649761-24-0 , entecavir ep impurity f | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
1)Supply capacity:10mg,,25mg,50mg
2)Delivery time: 7 days
3)Payment method:T/T
4)Packing:inner-glass bottle outer-box
5)Lead port:Shanghai.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
- President
- Song Xuepan
- Address
- No.176,Hangzhou West Road, Xialu Area,Huangshi,Hubei
- Product Category
- Chemical Reagents & Products
- Year Established
- 2015
- No. of Total Employees
- 1-50
- Company introduction
- Main Markets
-
 Brazil
Brazil
 Canada
Canada
 India
India
 Italy
Italy
 Mexico
Mexico
 Romania
Romania
 South Africa
South Africa
 South Korea
South Korea
 U. Kingdom
U. Kingdom
 U.S.A
U.S.A
- Main Product
Related Products

Limonin
Betamethasone 17-valerate

GBL Gamma-Butyrolactone USA Canada 99.9% Wheel Cleaner
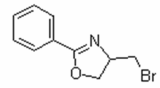
Cas 160149-03-1
16-beta Methyl Epoxide


































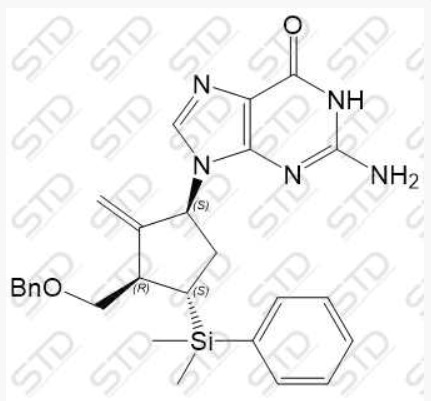
 China
China



