PneumoPatho 13 Detection Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- pneumonia, pnuemopatho, pp13, pcr kit for diseases

SOLGENT CO., LTD.
- Membership
- VIP
- Recent Visit
- Oct 25, 2024
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
16



| Product name | PneumoPatho 13 Detection Kit | Certification | - |
|---|---|---|---|
| Category |
Other Chemicals
Chemical Reagents & Products Pharmaceutical Intermediates |
Ingredients | - |
| Keyword | pneumonia , pnuemopatho , pp13 , pcr kit for diseases | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
PneumoPatho 13 Detection Kit
Real-Time PCR based assay system for simultaneous detection of pneumonia species
Pathogen Information
Pneumonia is usually caused by infection with viruses or bacteria, and less commonly by other microorganisms, certain drugs and other conditions such as autoimmune diseases. Pneumonia affects approximately 450 million people globally per year, seven percent of the global population, and results in about 4 million deaths per year, mostly in third world countries.
Product Specification
Detection target |
Set I : M. pneumoniae (MP) K. pneumoniae (KP) C. pneumoniae (CP) Set II : S. pneumoniae (SP) S. aureus (SA) L. pneumophila (LP) Set III : P. aeruginosa (PA) M. catarrhalis (MC) B. pertussis (BP) Set IV : H. influenza (HI) A. baumannii (AB) M. tuberculosis/ M. avium (TB/AV) |
Detection technology |
Real-Time PCR |
Specimen type |
Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage (BAL), Nasal swab, Oropharyngeal swab, Sputum |
Analytical sensitivity |
10 - 100 copies |
Compatible instruments* |
ABI 7500 / 7500 Fast Real-Time PCR System (Applied Biosystems) CFX96™ Real-Time PCR System (Bio-Rad) |
PCR running time |
~ 2 hrs |
* Please inquire us for compatible instrument information before use.
Product Features
- HotStart PCR system : Ultra high specific and sensitive result
- UDG system : No carryover contamination
- Multiplex PCR system : Multiple targets in a single reaction
- Reliable system : Automatic PCR control
- Positive control included
- Easy-to-use master mix
Reference
1. Micheal J McConnell et al. Quantitative Real-Time PCR for Detection of Acinetobacter baumannii Colonization in the Hospital Environment. Journal of Clinical Microbiology p. 1412-1414.
2. Ko WC, Paterson DL, Sagnimeni AJ, et al. Community-acquired Klebsiella pneumonia bacteremia: global differences in clinical patterns. Emerg Infect Dis 2002; 8:160-6.
3. Gierczynski R, Jagielski M, Rastswicki W, Klauzewski S (2007) Multiplex-PCR assay for idenrification of Klebsiella pneumonia isolates carrying the chain loci for K1 and K2 capsule biosynthesis. Pol J Microbiol 56: 153-156.
Result
Ordering Information
Technology |
Cat. No. |
Product |
Contents |
Real-Time PCR |
SQD81-K020 (20 reaction) |
DiaPlexQ™ PneumoPatho 13 Detection Kit |
2X Multiplex Real-Time PCR Smart mix (with UDG) (PneumoPatho 13) Primer & Probe Mixture I (PneumoPatho 13) Primer & Probe Mixture II (PneumoPatho 13) Primer & Probe Mixture III (PneumoPatho 13) Primer & Probe Mixture IV (PneumoPatho 13) Control Template I (PneumoPatho 13) Control Template II (PneumoPatho 13) Control Template III (PneumoPatho 13) Control Template IV (PneumoPatho 13) Nuclease free Water |
SQD81-K100 (100 reaction) |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- MYEONG, HYEON-KOON
- Address
- 32, 3F Techno6ro Yuseong-gu Daejeon
- Product Category
- Other Chemicals
- Year Established
- 2000
- No. of Total Employees
- 51-100
- Company introduction
-
SolGent InC, established in 2000, is a technuical market leader in the field of molecular biology. Based on the research ecperiences in KAIST(Korea Advanced Institute of Science and Technology), we are launching the leading products which meet the unremitting needs of life science. Our major products are PCR related thermostable ploymerases, DNA ligase, RNA plymerases, Reverse Tranx-x-scriptase, and DNA ligase RNA polymerases, Reverse Transcriptase, of genetic analysis, we are also providing the sequencing and genotyping services. The products and services are so competitive in the quality as well as the price. Especially, we are proud of the recently developed novel products, SEQrooge and H-Taq. You can find them here and enjoy their performances. Thank you
- Main Markets
-
 Canada
Canada
 China
China
 France
France
 Germany
Germany
 Philippines
Philippines
- Main Product
Related Products

glucolactone/Glucono-δ-lactone(GDL)

Lubricant(Engine Oil), Fully Synthetic, Synthetic, Mineral

M-Terphenyl
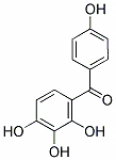
2,3,4,4'-Tetrehydroxybenzophenone, CAS NO.: 31127-54-5

Maleic anhydride