Avellino Corneal Dystrophy (ACD) Genotyping Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable

SOLGENT CO., LTD.
- Verified Certificate
-
17



| Product name | Avellino Corneal Dystrophy (ACD) Genotyping Kit | Certification | - |
|---|---|---|---|
| Category |
Other Chemicals
Chemical Reagents & Products Pharmaceutical Intermediates |
Ingredients | - |
| Keyword | molecular diagnostic , acd , avellino dystyophy , 2x multiplex pcr | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
Avellino Corneal Dystrophy (ACD) Genotyping Kit
Real-Time PCR (or Multiplex allele-specific PCR) based assay system for the genotyping of the ACD gene SNP related to avellino corneal dystrophy
Genetic information
ACD is a hereditary disease and one of the corneal dystrophies involving the formation of corneal opacities on different Layers of the corneal stroma, which leads to significant impairment of the corneal transparency and refraction. ACD is caused by the formation and deposition of abnormal hyaline protein which is generated by the replacement of histidine from arginine due to the mutation of cordon 124 (exon 4) in the βigh 3 gene. The deposited abnormal hyaline protein is usually known as kerato-epithelin and forms a granular, lattice precipitate causing visual impairment and might lead to blindness if it remains undiagnosed. Genetic mutation is highly accelerated after laser eye surgery in those people who have ACD, causing a worsening in vision rather than improvement after surgery. The awareness of ACD has developed across the world, with some opticians now refusing access to LASIK in patients with ACD due to the harmful effects the
procedure has on patients.
Product Specification
|
DiaPlexQ™ Avellino Corneal Dystrophy (ACD) Real-Time PCR Genotyping Kit
|
DiaPlexC™ Avellino Corneal Dystrophy (ACD) PCR Genotyping Kit
|
Detection target |
SNP of R124 (βigh3 coding gene codon 124, exon4) |
|
Detection technology |
Real-Time PCR |
Conventional (End-point) Multiplex PCR |
Specimen type |
Blood, Buccal epithelial cell, Hair (root) |
|
Compatible instruments* |
ABI 7500 / 7500 Fast Real-Time PCR System (Applied iosystems) CFX96™ Real-Time PCR System (Bio-Rad) |
ABI Veriti thermal Cycler (Applied
Biosystems) recommended |
PCR running time |
~ 1hr 30 min |
~ 1hr 30 min |
* Please inquire us for compatible instrument information before use.
Product Features
- HotStart PCR system : Ultra high specific and sensitive result
- UDG system : No carryover contamination
- Multiplex PCR : Multiple targets in a single reaction
- Reliable system : Automatic Internal control (DiaPlexC ™)
- Positive control included
- Easy-to-use master mix
- CE certification
Reference
1. Kocak-Atlintas AG, Kocak-Midillioglu I, Akarsu AN, Duman S. βigh gene analysis in the different diagnosis of corneal dystrophies. Cornea 2001; 20: 64-8.
2. Klintworth GK. Advances in the molecular genetics of corneal dystrophies. Am J phthalmol 1999; 128: 747-54.
3. Konishi M, Mashima Y, Nakamura Y, et al. Granular-lattice (Avellino) corneal dystrophy in Japanese patients. Cornea 1997; 16: 635-8.
Result & Data interpretation
Ordering Information
Technology |
Cat. No. |
Product |
Contents |
Real-Time PCR |
SQH26-K020 (20 reaction) |
DaPlexQ™ Avellino Corneal Dystrophy (ACD) Real-Time PCR Genotyping Kit |
2X Multiplex Real-Time PCR Smart mix (ACD) Primer & Probe Mixture (ACD) A/A type Control DNA (ACD) G/A type Control DNA (ACD) G/G type Control DNA (ACD) Nuclease free Water
|
SQH26-K100 (100 reaction) |
|||
Conventional (End-point) PCR |
SHG06-K020 (20 reaction) |
DiaPlexC™ Avellino Corneal
Dystrophy (ACD) Genotyping Kit |
2X Multiplex PCR Smart mix (with UDG) (ACD) Primer Mixture (ACD) Standard Marker (ACD) Wild type Control (ACD) Mutant type Control (ACD) Nuclease free Water |
SHG06-K100 (100 reaction) |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- MYEONG, HYEON-KOON
- Address
- 32, 3F Techno6ro Yuseong-gu Daejeon
- Product Category
- Other Chemicals
- Year Established
- 2000
- No. of Total Employees
- 51-100
- Company introduction
-
SolGent InC, established in 2000, is a technuical market leader in the field of molecular biology. Based on the research ecperiences in KAIST(Korea Advanced Institute of Science and Technology), we are launching the leading products which meet the unremitting needs of life science. Our major products are PCR related thermostable ploymerases, DNA ligase, RNA plymerases, Reverse Tranx-x-scriptase, and DNA ligase RNA polymerases, Reverse Transcriptase, of genetic analysis, we are also providing the sequencing and genotyping services. The products and services are so competitive in the quality as well as the price. Especially, we are proud of the recently developed novel products, SEQrooge and H-Taq. You can find them here and enjoy their performances. Thank you
- Main Markets
-
 Canada
Canada
 China
China
 France
France
 Germany
Germany
 Philippines
Philippines
- Main Product
Related Products
_2.jpg)
Ceramic Queen(Superhydrophobic Nano-Ceramic Coating for car)
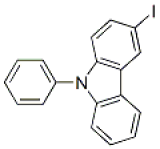
3-Iodo-N-phenylcarbazole, CAS NO.: 502161-03-7

3-amino-2-methylamino-5-(trifluoromethyl)pyridine
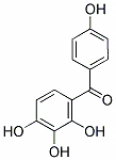
2,3,4,4'-Tetrehydroxybenzophenone, CAS NO.: 31127-54-5

Micro Slack Wax WX-160S, Microcrystalline Wax



































 South Korea
South Korea