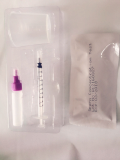
Mycoplasma cultivation/Identification/Susceptibility Test
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Brand name
- MB
- Payment Terms
- MoneyGram,Others,T/T,Western Union
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable

Mex Biotech Hong Kong Ltd
- Verified Certificate
-
12

| Product name | Mycoplasma cultivation/Identification/Susceptibility Test | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Test Kit Other Monitoring & Diagnostic Equipment |
Ingredients | - |
| Keyword | rapid test , mycoplasma test , mycoplasma , mycoplasma susceptibility test | Unit Size | - |
| Brand name | MB | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
Mycoplasma cultivation/Identification/ Enumeration/ Susceptibility Test Kit is intended for cultivation, identification, enumeration and antibiotic susceptibility tests of Ureaplasma Urealyticum (UU) and Mycoplasma Hominis(MH) in human urogenital tract in one step.
Mycoplasma is one of the main pathogens which lead to nongonoccal urethritis (NGU), cervical, pelvic inflammatory disease, such as orchitis, epididymitis etc, and cause infertility to men and women. These pathogens can attack and destroy urogenital epithelial cells, causing infection of AIDS and other transmitted diseases. Among all the Mycoplasma species, UU and MH are able to lead transmitted diseases. The incidence is continuously increasing, seriously compromising human health. The key to the treatment and prevention of the spread of Mycoplasma is timely and accurate detection. Currently, culture method is still being recognized as a reliable method to identify Mycoplasma infection.
The test kit consists of susceptibility culture media (freeze-dried) and IES test boards. The ingredients of culture media contain peptone, yeast extract, serum, growth factors, other nutrients, and urea, arginine. Urea can be decomposed by urease in UU and releases NH3; arginine can be decomposed by arginase in MH and releases NH3. NH3 increases the pH value of the culture broth and broth color turns from yellow to red. The broth contains antibacterial agent which inhibits the growth of bacteria and fungi. The test board's wells are designed for control test and UU, MH identification, enumeration and susceptibility test of UU and MH.
1.Repetition Rate: 100%
2.Coincidence Rate: more than 90%
3.Specificity: 100%
4.Intra-batch Discrepancy:less than 10%
5.Inter-batch Discrepancy: less than 5 %
6.Culture broth should be sterile and clear.
Package
1)Neutral bulk package;
2)Neutral box package;
3)OEM package.
Delivery Terms
1.We will ship the items after the payment is reached.
2.We can ship to you by UPS/DHL/TNT/Fedex. Pls contact us directly and we will use your preferred ways.
3.We are not responsible for any accidents, delaysor other issues that are the responsibility of the shipping service.
4.Any import fees or charges are the buyer's responsibility.
Male: Collect urethral secretions, prostate secretion, seminal fluid (liquefied first, then add 500ul ) or midstream urine for culture (for midstream urine, take 10ml for centrifugal at 2000r/min for 10 minutes, then collect the sediment 500ul for inoculation);
Female: Collect cervix secretion with help of speculum or amniotic fluid for culture. Urine is not recommended for culture.
For positive culture broth obtained by cultivating in normal mycoplasma medium, just suck up 30-50ul for test.
1.Ideal test result will be likely to obtain following the procedures above completely.
2.The microbiological inspection mainly depends on the collection of specimen, so a negative may not indicate that there is no infection of mycoplasma.
3.If a negative result is obtained, it only indicates mycoplasma may not exist in the medium.
4.For definite clinical diagnosis, it is necessary to appeal to other methods and instruments
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | MoneyGram,Others,T/T,Western Union | Shipping time | Negotiable |

- President
- Encore
- Address
- Room 1017 Tongxinyuan Liantang Luohu Shenzhen Guangdong
- Product Category
- Medical Devices,Medical Test Kit,Other Monitoring & Diagnostic Equipment
- Year Established
- 2001
- No. of Total Employees
- 51-100
- Company introduction
-
Mex Biotech Hong Kong Ltd.is a growing supplier for in-vitro diagnostics product.Develop,manufacture and distribute in-vitro rapid diagnostic test products.We have developed long-term business relationships with clients all over the world.Honesty is a basic principle of our company. Our company is committed to providing high quality products to customers, to customers as the center, do our best to meet customers'demands.Our aim is to satisfy our Long-term cooperation customers and welcome new clients.
We are currently providing an extensive range of immunochromatography based one-step rapid tests as below:
1)Fertility Hormone for HCG,LH ,FSH;
2)Infectious diseases & STD for HBsAg, Anti-HBsAb, HCV, HIV 1/2, HIV saliva,Syphilis, Malaria, dengue, H.pylori,TB,Chlamydia,Conorrhea,Typhoid,Rotavirus, Adenovirus;
3)Tumor markers for AFP, CEA, PSA, FOB;
4)Cardiac markers for Troponin I & Cardiac 3 in 1 (Troponin I, Myoglobin, CK-MB);
5)Urine reagent rapid test ,such as URS 1-12 parameters .
We appreciate for your interest in our products and looking forward more cooperation in near future.
- Main Markets
-
 Malaysia
Malaysia
 Myanmar
Myanmar
 Philippines
Philippines
 Saudi Arabia
Saudi Arabia
 Singapore
Singapore
 South Korea
South Korea
 Sri Lanka
Sri Lanka
 Turkey
Turkey
- Main Product
Related Products

Hairview

Catch Me Patch Her's Band

Pentagon Grand (CO2 fractional laser)

DENCLE ANDANTE FUNCTIONAL TOOTHBRUSH_10 Pieces

INCYTO Needle


































 China
China