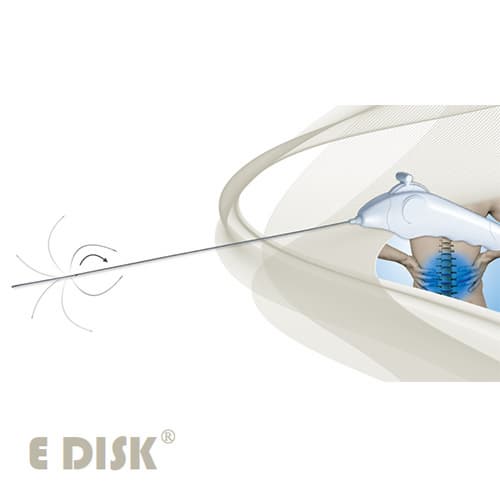
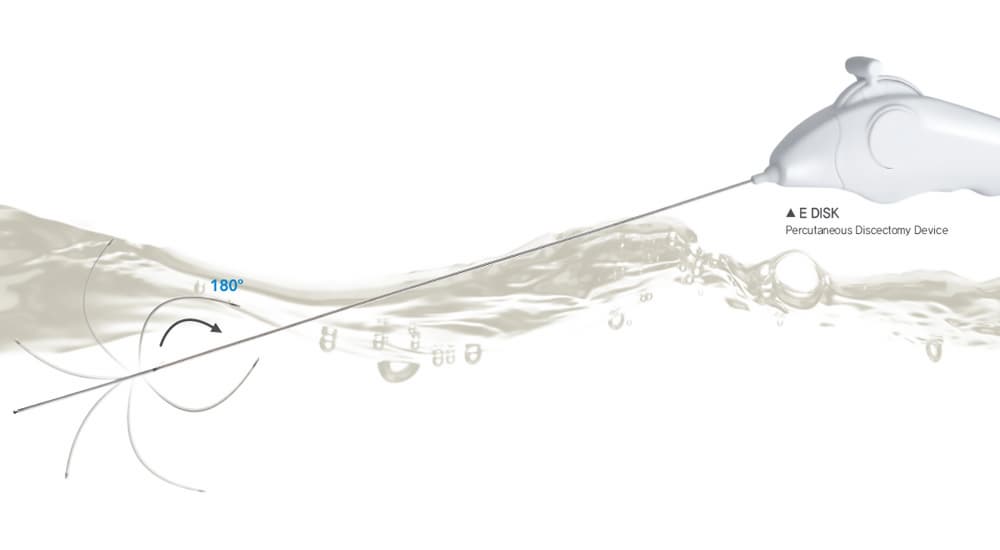
E Disk
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- cervical, lumbar, e-disk, pain releasepain releasepain release
- Category
- Medical Devices , Medical Consumables
OK MEDINET KOREA Co.,Ltd.
- Verified Certificate
-
12


| Product name | E Disk | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Consumables |
Ingredients | - |
| Keyword | cervical , lumbar , e-disk , pain releasepain releasepain release | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
E Disk
│ Description │
Requirement of the device managing pain effectively without spinal surgery
-Requirement of single used sterilized device.
-Requirement of direction control electrode in order to approach to the herniated nucleous pulposus.
│ HERNIATED DISC SYMPTOMS │
│ A Sterilized cable is provided │
│ TREATMENT PROCESS │
│ INDICATION │
-Hemiated nucleus pulposus
-Discogenic Pain
-Contained disk herniations
-Hemiated Disk Degeneration
│ ADVANTAGES │
-Short procedure and low risk therapy
-Local Anesthesia
-Minimal invasive and minimal defecton Patient's musculo skeletal structure
-The day of admission adn discharge
-Treatment of 1 hour
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Youn Moon, Jung
- Address
- Namseong Plaza 418,Digital-ro 130, Geumcheon-gu, Seoul, Korea
- Product Category
- Beauty & Personal Care,Medical Consumables,Medical Devices
- Year Established
- 2002
- No. of Total Employees
- 1-50
- Company introduction
-
OK Medinet Korea Co., Ltd., established in March 2002 mainly with the goal of providing excellent medical devices to be usedmainly for Orthopedic & Neurosurgery through co-operational research and development with famous Doctors in Korea University Hospital. Especially, we are manufacturing spinal disc treatment devices, PCM(Porous Coated Motion) and various medical devices for craniectomy by making continuous research and development most suitable for the required patients. With our brand and OEM brand, we are expanding to overseas market at the strength of internationally certified CE Mark and researched subjects.
Our current main products are as follows
* Orthopedic Cement Dispenser
Injector,OTN System Ky Toolkit, VP Needle, Kamma Knife, Osteo Toolkit Set
* Catheter Ballon, Kyphoplasty
Kyphoplasty Ballon System
* Spinal Internal Fixation System, Intervertebal Body
Pedicle Screw Spinal Fixation System
* Spinal Cage
HELIX
* Trephine Instrument, Manually Operated Single-Use
OKN
* Electrosurgical System Handpiece Foot Controlled, Single Use
E-Disk
Bone Cement
- Main Markets
-
 Iran
Iran
 Malaysia
Malaysia
 Turkey
Turkey
 U.S.A
U.S.A
- Main Product
Related Products
Bone Expander Kit Price

Covid 19 Detection

Catch Me Patch Her's Band

Dental Implant Ratchet Hex Drivers
MINIPIN; Home Beauty Device for anti-aging from Microneedles


































 South Korea
South Korea



