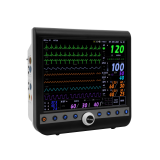
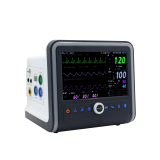
Patient Monitor VP-1000
Votem's Pre-configured patient monitor
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Multi-Parameter Monitor
| Product name | Patient Monitor VP-1000 | Certification | - |
|---|---|---|---|
| Category | Multi-Parameter Monitor | Ingredients | - |
| Keyword | medical device , medical equipment , patient monitor , vital sign monitor | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 1 |
| Supply type | Available | HS code | 9018197000 |
Product Information
Patient Monitor VP-1000
│ DESCRIPTION │
Patient Monitor is an essential medical equipment analyzing the various vital signs of patient and showing waveform and numeric types of data to medical professionals so that they can apprehend the condition of animal patients.
│ CONFIGURATION │
│ FEATURES │
B2B Trade
| Price (FOB) | Negotiable | transportation | Express,Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Tony Kang
- Address
- Dongnae-myeon, Geodudanji 1-gil,27, Chuncheon-si, Gangwon-do, Korea
- Product Category
- Multi-Parameter Monitor,Oximeter
- Year Established
- 2007
- No. of Total Employees
- 1-50
- Company introduction
-
VOTEM is manufacturing Patient Monitor (for Human and Veterinary) and other medical products at own factory site, supplying these products to the countries in Europe, America, Asia, and other continents.
VOTEM has established in 2007 by research personnel with masters or higher degree in its related fields of study to create the new value in the medical device market.
VOTEM will focus all activities to satisfy the needs of the customers through the value innovation. Also, VOTEM is planning to lead the medical industry by carrying out multiple research projetcts and clinical studies, establish and maintain the quality system in accordance with ISO13485 and Medical directive, 93/42/EEC.
- Main Markets
-
 Australia
Australia
 Malaysia
Malaysia
 Philippines
Philippines
 Romania
Romania
 U.A.E.
U.A.E.
- Main Product
Related Products

Urine Analyzer AS 300

Urine Analyzer AS 720
Multi Parameter Patient Monitor_IP4050
M50 Patient Monitor

Animal Urine Analysis Test Strip Self-Stik VET



































 South Korea
South Korea