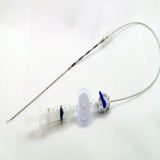
EDEN™ EndoCath Catheter(Guide Catheter for Epiduroscopy)
Back Pain Management
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Devices , Other Health Care Products
JMT Co., Ltd.
- Verified Certificate
-
16




| Product name | EDEN™ EndoCath Catheter(Guide Catheter for Epiduroscopy) | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Other Health Care Products |
Material | - |
| Keyword | minimally invasive procedure , direct lysis of scarring , chronic sciatica , low back pain | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 100 |
| Supply type | Available,OEM | HS code | 9018392000 |
Product Information
EDEN™ EndoCath Catheter(Guide Catheter for Epiduroscopy)
│Description│
Epiduroscopy is a less invasive procedure than traditional surgeries performed on the lower back. Not only procedure has been successful in helping to relieve some instances of chronic sciatica, it has also proven to be effective in cases where traditional epidurals and nerve root blocks have failed.
An epiduroscopy is typically performed as a day procedure and the patient is usually awake and can communicate with the doctor. After light sedation and a local anaesthetic are administered, a very small incision is made and a fibre optic camera is inserted in the lower back and guided up towards the affected nerve roots. Thanks to the small camera and miniscule tools, various treatment can be made effectively.
│Specification│
- Working Channel: 1.0mm
- Working Length: 270~300mm
- Tube Diameter: 3.0mm
│Indication│
• Observation of pathology and anatomy
• Direct medications application
• Direct lysis of scarring
• Placement of catheter and electrode systems
• An adjunct to minimally invasive surgery
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
- President
- Jeon Sangki
- Address
- Baekseok-eup, Gwonyul-ro 1203 beon-gil,70-39, Yangju-si, Gyeonggi-do, Korea
- Product Category
- Orthodontic Supplies
- Year Established
- 2006
- No. of Total Employees
- 1-50
- Company introduction
-
We are one of manufacturer and distributor in Korea producing the medical products using our manufacturing facilities. Our major products are Epidural Catheter for spinal pain management & PEEK cages for PLIF/ALIF. In order to diversify our existing market, we are interested in supplying our high quality products to you on favorable terms.
- Main Markets
-
 Mexico
Mexico
 Viet Nam
Viet Nam
- Main Product
- Attached File


































 South Korea
South Korea