DiaPlexQ Avellino Corneal Dystrophy ACD Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Clinical Analytical Instrument
EuDiPia Co., Ltd.
- Verified Certificate
-
11


| Product name | DiaPlexQ Avellino Corneal Dystrophy ACD Kit | Certification | - |
|---|---|---|---|
| Category | Clinical Analytical Instrument | Ingredients | - |
| Keyword | detection kit , genotyping kit , dystrophy acd genotyping kit | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
DiaPlexQ Avellino Corneal Dystrophy ACD Genotyping Kit
│ DESCRIPTION │
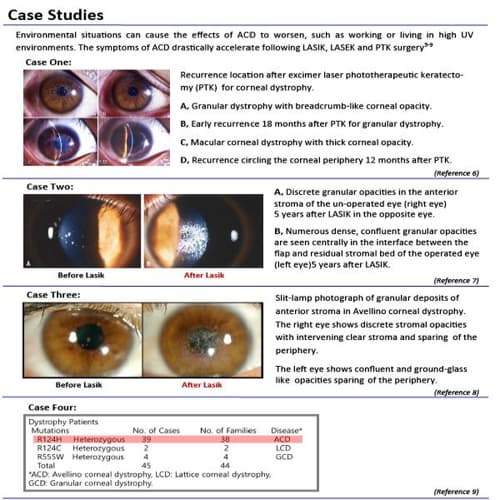
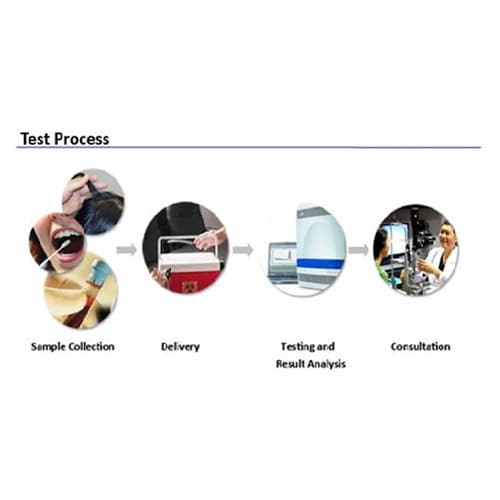
The DiaPlexQ Avellino Corneal Dystrophy (ACD) Genotyping Kit is designed to screen the single nucleotide polymorphism (SNP) of codon 124 (exon 4) of the βigh 3 gene responsible for Avellino Corneal Dystrophy, using Real-Time allele-specific PCR.
- Detection Technology: Real-Time PCR
- Specimens: Blood, Buccal epithelial cell, Hair (root)
- Target Organisms: SNP of R124H (βigh3 coding gene Codon 124, exon4)
- Analytical Sensitivity: 10-102 copies
│ FEATURES │
- HotStar PCR system: ultra high specific & sensitive result
- UDG system : no carryover contamination
- Reliable system: confirmation by control template
- Easy-to-use master mix: just add the template and primer
│ CONTENTS │
- 2X Multiplex Real-Time PCR Smart mix
- Primer & Probe Mixture
- A/A type Control DNA
- G/A type Control DNA
- G/G type Control DNA
- Nuclease free Water
│ SPECIFICATION │
- Compatible Real-Time Thermal Cyclers: ABI 7500, 7500 Fast, Bio Rad CFX96™
- Size:100 test - Cat. No.SQH26-K100, 20 test - Cat. No.SQH26-K020
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Kim Insoo
- Address
- #506,Venture Research Center,194-41 Osongsaengmyeong 1-ro, Osong-eup, Chungweon-gun,Chungcheongbuk-do,363-951,korea
- Product Category
- Medical Devices
- Year Established
- 2012
- No. of Total Employees
- 1-50
- Company introduction
-
☞ EuDiPia has established the headquarters and a research institute at the business center in Sogang University with a motto of “Diagnostics for Utopia” in September, 2012, and acquired certificate as a venture company from the KOTEC in April, 2013 and in January, 2014 moved into OSONG Medical Innovation Foundation. In June, 2014 EuDiPia has acquired KGMP(Korea Good Manufacturing Practice).
☞ EuDiPia possesses technology named One-tube Nested Multiplex real-time PCR, and the first product using that technology is the EuDxTM-MTB(Mycobacterium tuberculosis). And that has 100 times higher sensitivity compare to threshold cycle value of conventional real-time PCR products.
☞ “EuDxTM-MTB detection” that was registered with trade mark in April, 2013, that a company in partnership, Solgent Co., Ltd. has been consigned for the production of it. After acquired approval from MFDS(Ministry of Food & Drug Safety) in June, 2014 via proceeding of clinical trial in Seoul Asan Hospital, it is scheduled to provide products to large scaled institutions including general hospitals, central laboratories, and Korean Institute of Tuberculosis etc. starting from July, 2014.
☞ Technologies and products to be developed in the future are in the field of respiratory infectious diseases first and then covering other infectious viruses like hepatitis and HPVs etc. . It is planned to make a fully automated POCT system that is with improved convenience for users and also accuracy.
☞ For entering to the market, local distributors are to be involved for Korean market. And in case of China, the joint corporation is planned to be established. In addition, it is scheduled to establish country-specific trade route for emerging markets in other foreign countries. Achieves sales amount of ten billion won within 5 years since the first product is launched.
- Main Product
Related Products

MEDISCOPE D
COVID 19 IgM / IgG RAPID KIT

POCT Fluorescent Immunoassay diagnostic System

WATER BATH

PREGABLE Ovulation Pregnancy Tests Kit with APP, SmileReader


































 South Korea
South Korea



