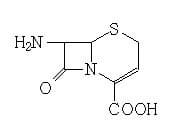
7-ANCA Intermediate for Ceftizoxime and Ceftibuten CAS no.36923-17-8
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Brand name
- Hipharma
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- antibiotics, cephalosporin, ceftibuten, ceftizoxime
- Category
- Pharmaceutical Intermediates
Hipharma Limited
- Country / Year Established
-
 China
/
China
/
- Business type
- Others
- Verified Certificate
-
11

| Product name | 7-ANCA Intermediate for Ceftizoxime and Ceftibuten CAS no.36923-17-8 | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | antibiotics , cephalosporin , ceftibuten , ceftizoxime | Unit Size | - |
| Brand name | Hipharma | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
7-ANCA
Chemical Name: 7-Amino-3-cephem-4-carboxylic acid
Molecular Formula: C7H8N2O3S
Formula Weight: 200.22
CAS No.: 36923-17-8
Mp: 215-218°C
Storage temp: -20°C Freezer
Chemical Properties: Off-White Solid
Usage: Intermediate for Ceftizoxime and Ceftibuten
Hipharma Limited is high-tech enterprise which sets R&D and production in the integration, specializing in chemical and pharmaceutical APIs and intermediates production and sales, especially in cephalosporin antibiotics field. We have the plant located in Shijiazhuang, hebei province.
At present, we are in a position to supply the following pharmaceutical raw material and intermediates:
1. Ceftizoxime Sodium sterile
2. Ceftizoxime Acid
3. Cefoxitin Sodium sterile
4. Cefoxitin Acid
5. Cefalotin Acid
6. Cefoperazone Sodium Sterile
7. Cefoperazone Acid
8. Cefepime Sterile with Arginine
9. Cefotiam Non sterile
10. Meropenem with Sodium Carbonate Anhydrous
11. Meropenem Non Sterile
12. Entecavir
13. 3-OH Main chain of Cephalosporin CAS no. 54639-48-4
14. 7-ANCA Main chain of Cephalosporin CAS no. 36923-17-8
15. 7-ADCA Main chain of Cephalosporin CAS no. 22252-43-3
If you have any question to our company or products, please feel free to contact us.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Joyce Wang
- Address
- Address: No. 188, Commercial Building of Xihuan Market, Kaitai Street, Qiaoxi District, Shijiazhuang, Hebei, 050081, China
- Product Category
- Beauty & Personal Care,Organic Intermediate,Pharmaceutical Intermediates
- No. of Total Employees
- 1-50
- Company introduction
-
Hipharma Limited is high-tech enterprise which sets R&D and production in the integration, specializing in chemical and pharmaceutical APIs and intermediates production and sales, especially in cephalosporin antibiotics field. We have the plant located in Shijiazhuang, hebei province. At present, we are in a position to supply the following pharmaceutical raw material and intermediates: 1. Ceftizoxime Sodium sterile 2. Ceftizoxime Acid 3. Cefoxitin Sodium sterile 4. Cefoxitin Acid 5. Cefalotin Acid 6. Cefoperazone Sodium Sterile 7. Cefoperazone Acid 8. Cefepime Sterile with Arginine 9. Cefotiam Non sterile 10. Meropenem with Sodium Carbonate Anhydrous 11. Meropenem Non Sterile 12. Entecavir 13. 3-OH Main chain of Cephalosporin CAS no. 54639-48-4 14. 7-ANCA Main chain of Cephalosporin CAS no. 36923-17-8 15. 7-ADCA Main chain of Cephalosporin CAS no. 22252-43-3 If you have any question to our company or products, please feel free to contact us.
- Main Product
Related Products
beta-cyclodextrin
Hydrocoralliaire- Klonopin- Lexapro
Betamethasone
16-beta Methyl Epoxide
Drug, medicine, API, pharmaceutical raw material, surgical dressing