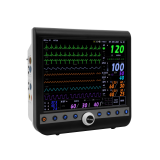
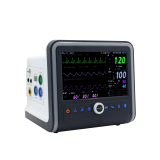
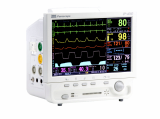
Central Monitoring System VC-2000
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Multi-Parameter Monitor
| Product name | Central Monitoring System VC-2000 | Certification | - |
|---|---|---|---|
| Category | Multi-Parameter Monitor | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Central Monitoring System VC-2000
VC-2000 central monitor is designed to assure a friendly operating environment as well as offering flexible and effective monitoring system environment to meet individual hospital needs.

Central System VC-2000
- Simultaneous monitoring up to 14 patients
- 7 day's (168 hours) graphical and numerical trend & event information
- Multi-waveform monitoring
- ECG (I, II, III, aVR, aVL, aVF, V1-V6), SpO2, RESP, 4 IBP, EtCO2
- Multi-Parameter monitoring
- HR, ST (I, II, III, aVR, aVL, aVF, V1-V6), PR, NIBP, PVC, SpO2,
- 4 TEMP, EtCO2
- LAN and Wireless LAN (AP : WDS Mode) Connection
- complete solution for LAN and wireless LAN networking
- simplified menu and easy to use interface
- bi-directional communication with VOTEM patient monitors
- large storage capacity of the waveforms and numeric information

 '
'



B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Tony Kang
- Address
- Dongnae-myeon, Geodudanji 1-gil,27, Chuncheon-si, Gangwon-do, Korea
- Product Category
- Multi-Parameter Monitor,Oximeter
- Year Established
- 2007
- No. of Total Employees
- 1-50
- Company introduction
-
VOTEM is manufacturing Patient Monitor (for Human and Veterinary) and other medical products at own factory site, supplying these products to the countries in Europe, America, Asia, and other continents.
VOTEM has established in 2007 by research personnel with masters or higher degree in its related fields of study to create the new value in the medical device market.
VOTEM will focus all activities to satisfy the needs of the customers through the value innovation. Also, VOTEM is planning to lead the medical industry by carrying out multiple research projetcts and clinical studies, establish and maintain the quality system in accordance with ISO13485 and Medical directive, 93/42/EEC.
- Main Markets
-
 Australia
Australia
 Malaysia
Malaysia
 Philippines
Philippines
 Romania
Romania
 U.A.E.
U.A.E.
- Main Product


































 South Korea
South Korea