TiO2 Photocatalyst
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- tio2 photocatalyst
- Category
- Other Chemicals

Nano Co., Ltd.
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
16



| Product name | TiO2 Photocatalyst | Certification | - |
|---|---|---|---|
| Category | Other Chemicals | Ingredients | - |
| Keyword | tio2 photocatalyst | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
1.Photocatalyst TiO2
Photocatalyst absorbs light and causes various chemical reactions. Metal oxide and sulfide are known for photocatalyst, and especially TiO2 is being widely commercialized due to high photocatalytic efficiency, chemical stability and wide range of application.
2.Reaction of TiO2 photocatalyst
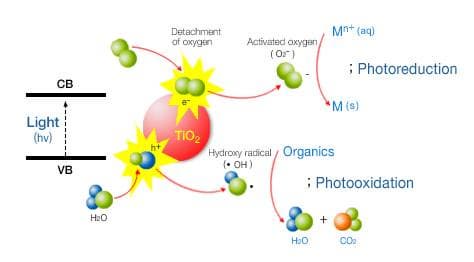
Under the condition of light energy which is bigger than band-gap energy(under 380nm) of the TiO2, it occurs photo-oxidation, photo-reduction and hydrophilic reaction. This reactivity can be used for a variety of uses. For example, the dissolution of hazardous VOCs, the removal of endocrine disruptors, the recovery of heavy metal, the reduction of NOx and SOx, antibiotics, sterilization, deodorization, anti-fogging, decontamination and self-cleaning.
- Mechanism of the decomposition of organics
- A hole(h+) and an electron(e-) occur respectively at VB(Valence Band) and CB(Conduction Band) by the irradiation of light, then hydroxy radical(ㆍOH) and activated oxygen(O2-) occur after reacting with water and oxygen in the atmosphere. As high reactivity of ㆍOH and O2-, hydroxy radical acts as a strong oxident and activated oxygen acts as a reductant. - Mechanism of hydrophilic reaction
- Hydrophilicity occurs when oxygen vacancy by the irradiation of light and the surface where organics are removed by photo-oxidation adsorb water.
3.Functions of a photocatalytic TiO2
The decomposition of organic contaminants: A
Photocatalytic TiO2 can decompose completely harmful organic contaminants which are hard to be decomposed using purification methods of chlorine or ozone. Therefore, it is very effective in decomposing and removing endocrine disruptors which are detrimental to environment and organic contaminants, despite existing small amounts.
- Organic chloride: Trichloroethylene
- Endocrine disruptors : Dioxin, Bisphenol, Nonylphenol, Estradiol
- Harmful VOCs: Acetaldehyde, Formaldeyhde, Toluene
- Odored gas: H2S, Thiol, Amine
- Other odor: Cigarette smell
- The decomposition of air contaminants: NOx, SOx, Dioxine from exhaust gas of cars, incinerators and power plants.
- Self-Cleaning: The dirt is removed naturally. Hydrophilicity makes dirt to be washed away by raindrop or water. The organic compounds on surface are decomposed by an oxidation reaction. These reactions unable dirt or organic compound to be accumlated on the TiO2 film.
- Anitbiosis & Sterilization: When UV light hits a photocatalytic TiO2, hydroxy radical(ㆍOH) occurs on surface and it kills bacteria. It means thatㆍOH even decompose the remains of bacteria and poisonous substances and other antibiotics although Ag and Cu do not show such a strong effect.

4.Applications of TiO2 photocatalyst

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- SHIN, DONG-WO
- Address
- 1188 Magong-ri, Cheongri-myeon, Sangjoo-si, Gyeongsangbuk-do, 660-882, Korea
- Product Category
- Other Vehicle Accessories
- No. of Total Employees
- 1-50
- Company introduction
-
Welcome to the ceramic specialty company. NANO is consistently pursuing leading company in ceramic raw material powder and extrusion products. We established a mass production line for nanoscale photocatalytic powder at the end of 2001. The new low-cost processing technology was certified by Ministry of Commerce, Korea, in July 2001. In addition, the pilot-plants for WS2 solid lubricant powder and ceramic honeycomb catalyst were also successfully completed in the early of 2001 after more than 3 years of intensive R&D. We will thus complete large-scale ceramic honeycomb production line in the middle of 2002.
Based on the our initial products of photocatalytic TiO2 and WS2 lubricants, we diversify our powder products for IT ceramic parts and ET ceramic honeycomb using our raw materials. Various applications of our raw materials such as self cleaning building materials and air purifying system will be presently shown in the market. NANO's key organization is R&D center consisting of 3 teams depending on the aimed processing , i.e., Raw Materials, Extrusion and Application. Our R&D activities mainly focus on developing the new low-cost processing.
I again appreciate you for your visit to NANO homepage. Your suggestions will be promptly responded.
- Main Product
Related Products

glucolactone/Glucono-δ-lactone(GDL)

Apolipoprotein E (ApoE) Genotyping Kit
B&B Baby Laundry Detergent

Power Dry-ux

M-Terphenyl