Fetal Monitor FM8000
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Fetal Heart Monitor

Trismed Co., Ltd.
- Verified Certificate
-
17



| Product name | Fetal Monitor FM8000 | Certification | - |
|---|---|---|---|
| Category | Fetal Heart Monitor | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Fetal Monitor
FM8000
- Light weight, easy operation
- 8.4" screen color LCD display, rotatable screen to 60°
- FHR 120-160bpm normal range label
- Manual/Automatic records fetal movement
- Sound and color alarm for high and low fetal hear rate
- Continuous 24-hour real-time monitoring function
- Continuous 12-hour patient curve and data storage, playback and print
- With picture freeze function
- Single, Twins Monitoring optional
- 9 crystal board band pulsed wave transducer
- Extra-long life, high-resolution built-in thermal recorder
- Built-in PC NET for the central monitoring system
Fetal Monitor FM8000(Type FM8000G) Technical Specifications
| FHR | ||
|
Measurement range
|
50-240bpm | |
|
Resolution
|
1bpm | |
|
Accuracy
|
±2bpm | |
|
Alarm setting
|
||
| Ultrasound probe | ||
|
Nominal Frequency
|
1.0MHz | |
|
Work Frequency
|
1.0MHz ±10% | |
|
Negative peak sound pressure
|
P<1MPa | |
|
Output beam intensity
|
Iob<20mW/cm | |
|
The peak time space peak intensity
|
Ispta<100mW/cm | |
|
The average time space peak intensity
|
Ispta<10mW/cm | |
|
FHR Rang
|
50BPM 240BPM | |
|
Resolution
|
1BPM | |
|
Accuracy
|
±2BPM | |
|
Record
|
Hospita, Bed No. Patient name, pregnancy weeks, Patient No. Paper speed, Data and time, FHR data and wave, Contraction data and wave, Fetal move times and mark etc. |
|
|
Display
|
Bed No. Pregnancy weeks, Age, Paper speed, Data and time, Volume, Alarm status, Transducer connection status, Recorder status, FHR data and wave, Contraction data and wave, Fetal move times and mark etc. | |
| TOCO | ||
|
Measurement range
|
0~100% | |
|
Resolution
|
1% | |
|
Non-linear error
|
Less than ±10% | |
|
Zero control
|
Manual | |
| Fetal Movement | ||
|
Fetal marking by manual button
|
||
| Display | 8.4" color TFT LCD 60degree folding type |
|
| Printer | ||
|
Paper
|
112mm/Thermal sensitive Z-fold type | |
|
Print Speed
|
10mm/min or 20mm/min or 30mm/min(optional) Ultrasonic Transducer |
|
|
Pulsed Doppler
|
||
|
Dimension
|
85mm x 6mm, 2m±0.5m | |
| Remote Marker | ||
|
Length
|
2m+1.2m | |
| PC interface | RJ45 | |
| Power | ||
|
AC
|
110~240VAC | |
|
Frequency
|
50/60Hz | |
|
Power(max)
|
60VA | |
|
Fuse
|
F1.6AL 240V | |
| Operating Environment | ||
|
Temperature
|
5~40°C | |
|
Relative Humidity
|
Less than 80% | |
|
Atmospheric Pressure
|
70~106kPha | |
| Transport and Storage | ||
|
Temperature
|
-10~55°C | |
|
Relative Humidity
|
Less than 80% | |
|
Atmospheric Pressure
|
70~106kPha | |
| Safety | ||
|
Class I type B
|
||
| Dimension | ||
|
Weight
|
About 3kg | |
| Size | 320(L) x 260(w) x 80(H)mm | |
| * Contents and specification subjects change without nitice | ||
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Hoon-Kyu Lee
- Address
- 409 SMECA, Yuseong-gu, Daejeon, Korea
- Product Category
- Other Examination & Testing Instrumnet
- Year Established
- 2014
- No. of Total Employees
- 1-50
- Company introduction
-
With company's philosophy of customer Satisfaction, Superiority and Specialization, TRISMED CO., LTD. was founded in 2000 and has been active in the research and development, manufacture and distribution on a world wide scale of cardiac diagnosis systems.
The wide-range products are targeted to offer reasonable price, international standard performance and high quality with our strong competence and deep experience.
TRISMED believes in coming true the leader in the cardiac diagnosis systems such as various Electrocardiographs(ECG), patient monitors and pulse oximeter in the world-wide.
Our quality system complies with the international quality standard - EN ISO13485:2003 and EC Council Directives 93/42/EEC CE(MDD).
- Main Markets
-
 Austria
Austria
 Romania
Romania
 Spain
Spain
 Turkey
Turkey
 U.S.A
U.S.A
- Main Product
Related Products

Pocket Size Ultrasound Fetal Doppler BT-200
Ultrasound Fetal Doppler BT-250
Doppler Fetal Monitor For Baby Heart Rate
Fetal & Maternal Monitor BT-380
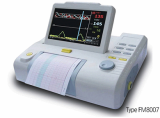
Fetal Monitor FM8000


































 South Korea
South Korea